If you’ve been following the energy curriculum up to this point and are looking forward to more great energy stuff, stay tuned. Electromagnetic energy is coming soon. However, before we get there, we need to take a bit of a side road. We need to wander into the world of quantum physics for a bit and take a look at the teeny and the mysterious world of atoms.We’re going to study atoms, their parts, as well as how they work together. Are you ready?
Select a Lesson
 | Special Science Teleclass: Chemistry This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too! (Click here if you’re looking for the more recent version that also includes Chemical Engineering.) When you think of slime, do you imagine slugs, … |
 | Electrolysis If you guessed that this has to do with electricity and chemistry, you’re right! But you might wonder how they work together. Back in 1800, William Nicholson and Johann Ritter were the first ones to split water into hydrogen and oxygen using electrolysis. |
 | Plasma Grape Be careful with this!! This experiment uses a knife AND a microwave, so you're playing with things that slice and gets things hot. If you’re not careful you could cut yourself or burn yourself. Please use care! |
 | Pile It In Density is basically how tightly packed atoms are. Mathematically, density is mass/volume. In other words, it is how heavy something is, divided by how much space it takes up. |
 | Quick & Easy Density Density is basically how tightly packed atoms are. (Mathematically, density is mass divided by volume.) For example, take a golf ball and a ping pong ball. Both are about the same size or, in other words, take up the same volume. |
 | Play With Your Food This is a simple experiment that really shows the relationship of mass, volume, and density. You don't need anything fancy, just a piece of bread. |
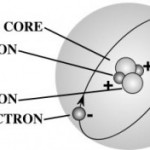 | Atomic Facts A gram of water (about a thimble of water) contains 1023 atoms. (That's a '1' with 23 zeros after it.) That means there are 1,000,000,000,000,000,000,000,000 atoms in a thimble of water! |
 | Lava Lamp We're going to watch how density works by making a simple lava lamp that doesn't need electricity! If you like to watch blob-type shapes shift and ooze around, then this is something you're going to want to experiment with. |
