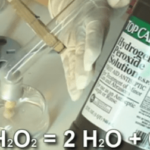Reducing Agent Orange
Today we will investigate the reducing power of orange. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 156, 157 Materials: potassium iodide potassium permanganate sodium carbonate test tube lid opener measuring spoon large graduated beaker two small graduated beakers vitamin C tablets 9V battery battery clip two alligator clips paper towel copper … Continue reading "Reducing Agent Orange" |
Potassium Hexacyanoferrate Reagent
Today we will be investigating potassium hexacyanoferrate as a detection agent for metals, specifically iron. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 15, 16, 17, 18 Materials: test tubes vial measuring spoon lid opener baking soda water hydrochloric acid pipette ammonium iron sulfate potassium hexacyanoferrate iron filings [/am4show] |
Introduction: Kit Overview, Tripod Assembly, Alcohol Burner Assembly, Insertion and Removal of Glass Tubing in Rubber Stopper
Welcome! Today we will be discussing tips on setting up your work station, and going over the contents in your chemistry kit. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: Introduction Part 1 [/am4show] |
Introduction: Household Items Needed and Chemicals to be Ordered
Today we will be gathering items needed that are not provided in the kit. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: Introduction Part 2 [/am4show] |
Silver Nitrate Reagent Part 1
Today we will learn another separation technique and how to use silver nitrate to detect chloride in a solution. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 19, 20, 21 Materials: test tubes grey stopper pipette funnel filter paper 1% silver nitrate sodium thiosulfate sand water table salt [/am4show] |
Synthesizing Copper Sulfate
Today we will be synthesizing and analyzing copper sulfate. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 10, 11, 12, 13, 14 Materials: sodium hydrogen sulfate copper sulfate iron filings test tube clamp test tube rack test tubes graduated beaker ammonia measuring spoon lid opener boiling rod copper sheeting alcohol burner steel … Continue reading "Synthesizing Copper Sulfate" |
Introduction: Solution Preparation
Today we will be diluting and preparing solutions. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: Introduction Part 3 [/am4show] |
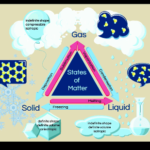
Phases of Water Part 1
Today we will be covering part 1 of the phases of water. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 23, 24, 25 Materials: test tubes grey stopper stopper with one hole pointed glass tubing test tube clamp scale matches or lighter graduated beaker crushed ice small jar Erlenmeyer flask (E flask) … Continue reading "Phases of Water Part 1" |
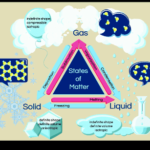
Phases of Water Part 2
Today we will be covering part 2 of the phases of water, and learning about distillation. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 26, 27, 28, 29 Materials: test tube test tube clamp test tube rack potassium hexacyanoferrate silver nitrate matches or lighter tripod jar table salt water Erlenmeyer flask alcohol … Continue reading "Phases of Water Part 2" |
Water Purification
Today we will be investigating how to purify water. [am4show have='p9;p52;' guest_error='Guest error message' user_error='User error message' ] C3000 Experiment: 37, 38, 39, 40, 41, 42, 43, 44 Materials: test tube lid opener measuring spoon test tube clamp test tube rack charcoal ammonium iron sulfate syringe paper funnel filter paper sand garden soil puddle water … Continue reading "Water Purification" |
Cooling Crystallization
Today we will investigate crystallization through cooling and how it can be used as a separation and purification technique. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 30 Materials: test tube ammonium chloride test tube rack test tube clamp water alcohol burner lid opener measuring spoon matches or lighter [/am4show] |
Electrolysis Part 2
Today we will investigate how to split water molecules with electricity in a process known as electrolysis. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 45 Materials: small graduated beaker beaker cardboard cutout alcohol burner stand one molar sodium hydroxide litmus solution battery clip two alligator clips two nails or screws 9V … Continue reading "Electrolysis Part 2" |
Gases
Today we will investigate air pressure and gases. [am4show have='p9;p52;' guest_error='Guest error message' user_error='User error message' ] C3000 Experiment: 46, 47, 48, 49, 50 Materials: test tube cardboard basin angled glass tubing stopper with one hole straw Erlenmeyer flask graduated beaker water [/am4show] |
Potassium Permanganate Experiments
Today we will be investigating potassium permanganate. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 72, 73, 74 Materials: denatured alcohol hydrogen peroxide baking soda funnel filter paper alcohol burner Erlenmeyer flask test tubes grey stopper lid opener measuring spoon test tube clamp boiling rod paper towels potassium permanganate sod hydrogen sulfate … Continue reading "Potassium Permanganate Experiments" |
Combustion
Today we will be studying combustion. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 63, 64, 65, 66, 67 Materials: matches litmus solution limewater Erlenmeyer flask basin wooden splint saucer tealight candle grey stopper straw pipette glass jar with lid white vinegar alcohol burner [/am4show] |
Hydrogen from Magnesium and Aluminum
Today we will produce hydrogen gas from magnesium and aluminum. [am4show have='p9;p52;' guest_error='Guest error message' user_error='User error message' ] C3000 Experiment: 53, 55, 56 Materials: test tubes test tube clamp two stoppers with one hole grey stopper magnesium strip one molar sodium hydroxide tripod clothespin, tweezers, or tongs (recommended) vinegar evaporating dish wire mesh alcohol … Continue reading "Hydrogen from Magnesium and Aluminum" |
Calcium Hydroxide Reagent
Today we will study calcium hydroxide and how it can used as a reagent. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 60, 61, 62 Materials: glass vial (optional, you can use graduated beaker instead) litmus solution calcium hydroxide Erlenmeyer flask grad beaker with lid white vinegar evaporating dish funnel filter paper … Continue reading "Calcium Hydroxide Reagent" |
Silverware Polishing
Today we will learn about polishing silver. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 189, 190, 191 Materials: iron sulfate grains (left over from a previous experiment) grey stopper test tubes measuring spoon lid opener ammonium chloride calcium hydroxide large glass jar hydrochloric acid table salt evaporating dish aluminum foil silver … Continue reading "Silverware Polishing" |
Potassium Permanganate and Hydrogen Peroxide
Today we will be investigating potassium permanganate and hydrogen peroxide. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 90, 91, 92 Materials: matches Erlenmeyer flask hydrogen peroxide solution potassium permanganate sodium hydrogen sulfate sodium carbonate vinegar tripod test tube clamp wooden splint test tubes basin syringe acute angled glass tubing pointed glass … Continue reading "Potassium Permanganate and Hydrogen Peroxide" |
Catalyst: Potassium Iodide (KI)
Today we will investigate how potassium iodide can be used as a catalyst. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 89 Materials: test tube lid opener measuring spoon sodium thiosulfate potassium iodide hydrogen peroxide wooden splint pipette matches [/am4show] |
Activated Charcoal
Today we will be studying activated charcoal. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 84, 85 Materials: matches activated charcoal measuring spoon lid opener test tube wooden splint funnel filter paper hydrogen peroxide [/am4show] |
Decomposing through Heavy Metals
Today we will be studying how hydrogen peroxide decomposes through heavy metals. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 86, 87, 88 Materials: two molar hydrochloric acid (HCI) one molar sodium hydroxide (NaOH) copper sulfate ammonium iron sulfate matches hydrogen peroxide measuring spoon lid opener wooden splint two pipettes test tubes … Continue reading "Decomposing through Heavy Metals" |
Iron Sulfide and Sulfur
Today we will be producing hydrogen sulfide and sulfur dioxide gases. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 187, 188 Materials: hydrochloric acid matches wire mesh alcohol burner alcohol burner stand uncoated sheet metal lid aluminum foil test tube measuring spoon lid opener sulfur iron filings [/am4show] |
Baking Soda
Today we will compare sodium carbonate (also known as soda), and sodium bicarbonate (which is known as baking soda). [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 220, 221, 222 Materials: test tubes lid opener measuring spoon sodium carbonate sodium bicarbonate (baking soda) pipettes red litmus paper grey stopper stopper with one … Continue reading "Baking Soda" |
Alkaline Earth Metals: Magnesium
Today we will be investing the alkaline earth metal: Magnesium. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 234, 235, 236, 237 Materials: hydrochloric acid sodium hydroxide test tubes grey stopper ammonium chloride magnesium strip vinegar pipette lid opener measuring spoon [/am4show] |
Copper Part 1
Today we will be covering part 1 in our investigation into copper. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 252, 253, 254, 255, 256, 257 Materials: copper sulfate sodium carbonate steel wool or iron filings white vinegar hydrochloric acid sodium hydroxide ammonia potassium hexacyanoferrate citric acid test tubes lid opener pipette … Continue reading "Copper Part 1" |
Hydrogen Chloride Part 2
Today we will be continuing our investigation of hydrogen chloride. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 111, 112, 113, 114, 115 Materials: sodium hydrogen sulfate test tube lid opener pipette measuring spoon grey stopper small graduated beaker table salt vinegar silver nitrate (1%) dilute hydrochloric acid ammonia aluminum foil 9V … Continue reading "Hydrogen Chloride Part 2" |
Electrolytes: Conductors or Non-Conductors? Part 2
Today we will be covering part 2 of our investigation of whether electrolytes conduct or don’t conduct electricity. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 102, 103 Materials: small graduated beaker carbon electrode lid opener measuring spoon copper sulfate sodium hydrogen sulfate two alligator clips 9V battery battery clip alcohol burner … Continue reading "Electrolytes: Conductors or Non-Conductors? Part 2" |
Hydrogen Chloride Part 1
Today we will be investigating hydrogen chloride. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 105, 106, 107, 108, 109, 110 Materials: baking soda table salt tripod alcohol burner stand small graduated beaker alcohol burner rubber tubing angled glass tubing stopper with one hole pipette boiling rod hydrochloric acid test tubes syringe … Continue reading "Hydrogen Chloride Part 1" |
Chlorine Part 1
Today we will cover part 1 of our chlorine investigation. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 117, 118 Materials: blue litmus paper sodium thiosulfate potassium permanganate small graduated beaker with lid lid opener measuring spoon test tubes hydrochloric acid [/am4show] |
Hydrogen Bromide
Today we will be producing and analyzing a solution of hydrogen bromide. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 128, 129, 130, 131, 132, 133 Materials: jar aluminum foil litmus solution silver nitrate potassium bromide sodium hydrogen sulfate sodium carbonate sodium thiosulfate baking soda test tube clamp grey stopper blue litmus … Continue reading "Hydrogen Bromide" |
Electrolytes: Conductors or Non-Conductors? Part 1
Today we will be doing part 1 of our investigation into electrolytes and discovering whether they are conductors or non-conductors. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 93, 94, 95, 96, 97, 98, 99, 100, 101 Materials: aluminum foil battery clip 9V battery two alligator clips activated charcoal sodium hydroxide hydrochloric … Continue reading "Electrolytes: Conductors or Non-Conductors? Part 1" |
Chlorine Part 2
Today we will be caovering part 2 of our investigation into chlorine. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 119, 120, 121 Materials: matches vial Erlenmeyer flask hydrochloric acid sodium hydroxide flour potassium iodide activated charcoal potassium permanganate sodium thiosulfate test tubes cotton balls (or paper towels) lid opener measuring spoon … Continue reading "Chlorine Part 2" |
Iodine
Today we will be investigation iodine. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 134, 135, 136, 137, 138, 139, 140 Materials: sodium hydroxide vial 9V battery red litmus paper potassium permanganate potassium iodide sodium thiosulfate sodium hydrogen sulfate test tubes test tube clamp denatured alcohol vinegar tripod alcohol burner battery clip … Continue reading "Iodine" |
Silver Nitrate Reagent Part 2
Today we will be covering part 2 of utilizing silver nitrate as a reagent. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 124, 125, 126, 127 Materials: test tubes sodium thiosulfate potassium iodide potassium bromide silver nitrate lid opener measuring spoon [/am4show] |
Starch-Iodine Complex
Today we will be learning about the starch iodine complex [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 141, 142, 143, 144, 145, 146 Materials: denatured alcohol jar matches alcohol burner test tubes lid opener measuring spoon pipette boiling rod sodium thiosulfate ethanolic iodine starch solution [/am4show] |
Redox Reactions of Halogens
Today we will be learning about redox reactions. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 147, 148, 149 Materials: starch solution chlorine water test tubes potassium iodide potassium bromide sodium thiosulfate measuring spoon lid opener [/am4show] |
Specters in Chemistry
Today we will witness the appearance and disappearance of a specter in our lab, and learn the chistry behind it. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 150, 151, 152, 153 Materials: hydrogen peroxide sodium hydroxide hydrochloric acid ammonium iron sulfate sodium hydrogen sulfate sodium thiosulfate potassium permanganate potassium iodide copper … Continue reading "Specters in Chemistry" |
Zinc Dust
Today we will be [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: Materials: [/am4show] |
Electrolysis of Halogenides
Today we will be [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 158, 159 Materials: pipette lid opener zinc sheet two alligator clips nail measuring spoon alcohol burner 9V battery battery clip small graduated beaker cardboard cutout for beaker alcohol burner stand potassium hexacyanoferrate sodium hydrogen sulfate sodium hydroxide copper wire [/am4show] |
Delayed Ignition with Potassium Permanganate
Today we will be investigation potassium permanganate and its role as an oxidizer. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 154, 155 Materials: test tubes sodium hydrogen sulfate potassium iodide potassium permanganate sodium hydroxide hydrogen peroxide prepared starch solution grey stopper pipette lid opener measuring spoon [/am4show] |
Electrochemical Reactions
Today we will be performing electrochemical analysis of metals. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 160, 161 Materials: potassium hexacyanoferrate 9V battery battery clip two alligator clips graduated beaker table salt nail penny aluminum foil paper towel [/am4show] |
Galvanic Cells
Today we will be learning about galvanic cells. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 163, 164, 165, 166, 167, 168 Materials: a few meters of insulated wire basin table salt red litmus paper small graduated beaker sodium hydrogen sulfate magnesium strip sodium hydroxide test tubes cardboard cutout for beaker alcohol … Continue reading "Galvanic Cells" |
Litmus with Acids and Bases
Today we will be investigating acids and bases using litmus solutions. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 169, 170, 171, 172, 173, 174 Materials: white vinegar sodium carbonate test tubes red litmus paper blue litmus paper sodium hydroxide vitamin C tablets citric acid limewater litmus solution pipette measuring spoon lid … Continue reading "Litmus with Acids and Bases" |
Copper Salts
Today we will be investigating copper salts. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 179, 180, 181 Materials: hydrochloric acid sodium carbonate copper sulfate stopper with one hole grey stopper test tube clamp boiling rod test tubes glass dish or beaker large graduated beaker Erlenmeyer flask evaporating dish wire mesh alcohol … Continue reading "Copper Salts" |
Acidic and Basic Salts
Today we will be studying acidic and basic salts. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 175, 176 Materials: test tubes litmus solution sodium carbonate sodium hydrogen sulfate red litmus paper measuring spoon lid opener [/am4show] |
Titration
Today we will be investigating titration. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 177, 178 Materials: tripod sodium hydroxide litmus solution Erlenmeyer flask white vinegar syringe rubber tubing glass bead hose coupler test tube clamp pointed glass tube white paper [/am4show] |
Desiccants
Today we will be learning about the desiccate calcium chloride. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 182,183 Materials: calcium hydroxide sodium hydrogen sulfate hydrochloric acid grey stopper test tubes evaporating dish wire mesh alcohol burner alcohol burner stand lid opener measuring spoon pipette [/am4show] |
Water of Crystallization
Today we will be learning about water of crystallization and the important role it play in the crystalline structure of molecules. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 184, 185, 186 Materials: matches test tubes test tube clamp copper sulfate measuring spoon lid opener denatured alcohol alcohol burner paper towel [/am4show] |
Sulfur Sulfate
Today we will be investigating sulfur sulfates, more commonly known as thiosulfates. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 197, 198 Materials: test tubes boiling rod lid opener measuring spoon test tube clamp grey stopper sodium thiosulfate matches hydrochloric acid alcohol burner pipette filter paper funnel [/am4show] |
Acid Rain
Today we will be investigating acid rain. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 192, 193 Materials: matches alcohol burner grey stopper lid opener burning spoon (measuring spoon) sulfur Erlenmeyer flask test tube clamp pipette blue litmus paper [/am4show] |
Halogen Reducing Agent
Today we will investigate the reducing effect of sodium thiosulfate. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 199, 200 Materials: measuring spoon pipette test tubes lid opener potassium permanganate sodium hydrogen sulfate sodium thiosulfate light colored fabric iodine solution [/am4show] |
Preparation of Iodine Solution
C3000 Experiment: 135 [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] Materials: two small graduated beakers potassium iodide measuring spoon lid opener carbon electrode copper strip 9V battery battery clip two alligator clips paper towel [/am4show] |
Ammonia
Today we will be investigating ammonia. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 201, 202, 203, 204, 205, 206, 207 Materials: tripod alcohol burner test tubes litmus solution test tube clamp ammonium chloride calcium hydroxide hydrochloric acid sodium hydroxide ammonia small graduated beaker with lid cotton ball evaporating dish sheet metal … Continue reading "Ammonia" |
Carbon Dioxide
Today we will be setting up a carbon dioxide generator and studying carbon dioxide [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 211, 212, 213, 214, 215 Materials: matches container at least 1.5″x3″ basin old plate test tubes wooden splint test tube clamp two stoppers white vinegar baking soda tripod limewater Erlenmeyer … Continue reading "Carbon Dioxide" |
Mineral Water
Today we will be learning about mineral water. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 216, 217, 218, 219 Materials: limewater litmus solution your carbon dioxide generator white vinegar fresh mineral water baking soda alcohol burner boiling rod test tubes pipette [/am4show] |
Carbon Dioxide Fountain & Furnace
Today we will be creating a carbon dioxide fountain and a carbon dioxide furnace. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 224, 225, 226 Materials: alcohol burner your carbon dioxide generator setup test tubes measuring spoon lid opener grey stopper limewater sodium hydroxide vinegar baking soda basin angled glass tubing two … Continue reading "Carbon Dioxide Fountain & Furnace" |
Aluminum
Today we will be experimenting with aluminum. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 238, 239, 240, 241, 242 Materials: carbon dioxide generator funnel filter paper alcohol burner sodium hydroxide hydrochloric acid aluminum foil baking soda vinegar test tubes pipette wood splint matches [/am4show] |
Flame Test for Chemicals
Today we will learn how to identify certain chemicals by how they burn. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 231, 232, 233 Materials: table salt calcium hydroxide copper sulfate hydrochloric acid sodium hydroxide alcohol burner evaporating dish pipette lid opener measuring spoon three paper clips three corks [/am4show] |
Zinc
Today we will be working with the transition metal zinc. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 243, 244, 245, 246, 247 Materials: sodium hydroxide hydrochloric acid ammonia bakers ammonia (salt of hartshorn) white vinegar matches litmus solution potassium hexacyanoferrate alcohol burner test tubes pipette test tube clamp measuring spoon zinc … Continue reading "Zinc" |
Iron
Today we will be investigating iron [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 248, 249, 250, 251 Materials: white vinegar iron filings ammonium iron sulfate test tubes hydrogen peroxide hydrochloric acid sodium hydroxide potassium hexacyanoferrate litmus solution matches alcohol burner pipette test tube clamp measuring spoon [/am4show] |
Copper Part 2
Today we will be covering part 2 in our investigation of copper. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 258 Materials: tripod white vinegar litmus solution test tubes test tube clamp calcium hydroxide ammonium chloride copper sulfate lid opener Erlenmeyer flask wire mesh alcohol burner alcohol burner stand steel wool or … Continue reading "Copper Part 2" |
Sliver
Today we will be investigating silver. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 259, 260, 261, 262, 263 Materials: matches white vinegar evaporating dish test tubes lid opener table salt distilled water sodium thiosulfate sodium hydroxide hydrogen peroxide ammonia silver nitrate measuring spoon pipette wooden splint [/am4show] |
Polar and Non-Polar Compounds
Today we will be learning about polar and non-polar compounds. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 284, 285, 286, 287 Materials: test tubes lid opener measuring spoon copper sulfate grey stopper lubricating oil cooking oil [/am4show] |
Hydrocarbons
Today we will investigate hydrocarbons, molecules made up on hydrogen and carbons. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 268, 269 Materials: test tubes litmus solution matches screw top jar with lid tealight candle old plate or saucer [/am4show] |
Unsaturated Fatty Acids
Today we will utilize the Bayer test to detect unsaturated fatty acids. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 288 Materials: test tubes potassium permanganate sodium carbonate grey stopper lid opener measuring spoon cooking oil [/am4show] |
Hydrogen and Halogens
Today we will be investigating hydrogen and halogens and how halogens can replace hydrogens in molecular compounds. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 266, 267 Materials: evaporating dish alcohol burner alcohol burner stand boiling rod test tubes potassium iodide sodium carbonate lid opener denatured alcohol 9V battery small graduated beaker … Continue reading "Hydrogen and Halogens" |
Splitting Molecules
Today we will be splitting molecules. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 271, 272 Materials: matches basin denatured alcohol test tubes small graduated beaker test tube clamp grey stopper tripod alcohol burner burner stand fine sand angled glass tubing red stopper with one hole rubber tubing bromine water [/am4show] |
Acetic Ester
Today we will prepare acidic ester, which is found in glue, and gives off a fruity touch to soft drinks and candies. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 281, 282, 283 Materials: vinegar sodium carbonate sodium hydrogen sulfate dry test tubes denatured alcohol evaporating dish wire mesh alcohol burner alcohol … Continue reading "Acetic Ester" |
Preparation of Bromine Water
[am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 270 Materials: potassium bromide measuring spoon lid opener large graduated beaker with lid two small graduated beakers paper towel 9V battery carbon electrode copper strip two alligator clips battery clip vial to put bromine water [/am4show] |
From Fat to Soap
Today we will be learning how soap is prepared from fats [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 289, 290 Materials: lubricating oil cooking oil distilled water boiling rod test tubes red stopper with hole test tube clamp sodium hydroxide tripod alcohol burner [/am4show] |
Soap and Hard Water
Today we will investigate the effect of hard water on different types of soaps. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 291, 292, 293, 294 Materials: alcohol burner dishwashing soap boiling rod test tubes grey stopper test tube clamp distilled water mineral water lime water bar of soap [/am4show] |
Soap and Acids
Today we will determine the effect of acid on soaps. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 295, 296 Materials: Vinegar Liquid Soap Distilled Water Bar of Soap Grey Stopper Test Tubes Pipettes [/am4show] |
Breaking Down Starch
Today we will be testing starch’s ability to reduce Fehling solution. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 316, 317 Materials: wire mesh alcohol burner alcohol burner stand glucose hydrochloric acid sodium hydroxide starch suspension solution (the one you created in a previous experiment) citric acid large graduated beaker with lid … Continue reading "Breaking Down Starch" |
Starch: Soluble and Insoluble
Today we will be investigating starch in its soluble and insoluble forms. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 312, 313, 314, 315 Materials: jar cornstarch potassium permanganate sodium hydrogen sulfate test tube clamp lid opener measuring spoon boiling rod test tubes tripod Erlenmeyer flask wire mesh alcohol burner alcohol burner … Continue reading "Starch: Soluble and Insoluble" |
Mirror in a Test Tube
Today we will be creating a mirror inside of a test tube. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 308, 309, 311 Materials: sugar glucose neutralized sugar solution (the one you saved from a previous experiment) boiling rod test tubes ammonia silver nitrate alcohol burner pipette [/am4show] |
Fehling’s Solutions Sugar Test
Today we will be utilizing the Fehling’s test to detect sugars. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 304, 305, 306, 310 Materials: sugar glucose copper sulfate boiling rod test tubes citric acid test tube clamp vinegar sodium hydroxide (NaOH) Erlenmeyer flask alcohol burner red litmus paper pipette lid opener measuring … Continue reading "Fehling’s Solutions Sugar Test" |
Surfactants
Today we will be learning about surfactants. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 294, 298, 299, 300, 301 Materials: basin test tubes large beaker cooking oil distilled water liquid soap bar of soap grey stopper pipette carbon electrode pieces of fabric including water repellent fabric [/am4show] |
Glucose
Today we will be working with glucose [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 307 Materials: copper sulfate boiling rod test tubes test tube clamp glucose alcohol burner sodium hydroxide pipette lid opener measuring spoon stopper [/am4show] |
Proteins
Today we will begin our investigation in proteins. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 321, 322, 323, 324, 325 Materials: salt Erlenmeyer flask copper sulfate boiling rod test tubes test tube clamp egg (specifically an egg white) grey stopper denatured alcohol sodium hydroxide hydrochloric acid large graduated beaker alcohol burner … Continue reading "Proteins" |
Detecting Proteins in Food
Today we will be utilizing the biuret reaction to detect proteins in various foods. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 326, 327, 328, 329, 330 Materials: large graduated beaker alcohol burner test tubes copper sulfate stopper test tube clamp red litmus paper Erlenmeyer flask hydrochloric acid sodium hydroxide milk raw … Continue reading "Detecting Proteins in Food" |
Catalase
[am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: 331, 332, 333 Materials: Alcohol Burner Matches Test Tubes Stopper Test Tube Clamp Dry Yeast Hydrogen Peroxide Measuring Spoon Wooden Splint Ground Beef [/am4show] |
Separating Mixtures
Today we will investigate how mixtures can be separated. [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error message’ ] C3000 Experiment: Materials: test tubes test tube rack test tube clamp evaporating dish wire mesh alcohol burner with lid alcohol burner stand sand measuring spoon two graduated beakers with lids matches table salt water ammonium chloride [/am4show] |
Basic Chemistry Safety Information
Chemical Data & Safe Handling Information Sheet What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW! When you’re done … Continue reading "Basic Chemistry Safety Information" |
Making Litmus Solution and Paper
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH. Every liquid has a pH. If you pay particular attention to this lab, you will … Continue reading "Making Litmus Solution and Paper" |
Magnesium Battery
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum … Continue reading "Magnesium Battery" |
Making Copper
In this lab, we’re going to investigate the wonders of electrochemistry. Electrochemistry became a new branch of chemistry in 1832, founded by Michael Faraday. Michael Faraday is considered the “father of electrochemistry”. The knowledge gained from his work has filtered down to this lab. YOU will be like Michael Faraday. I imagined he would have … Continue reading "Making Copper" |
Making Chlorine
If we don’t have salt, we die. It’s that simple. The chemical formula for salt is NaCl. Broken down, we have Na (sodium) and Cl (chlorine). Either one of these can be fatal in sufficient quantities. When chemically combined, these two deadly elements become table salt. What once could kill now keeps us alive. Isn’t … Continue reading "Making Chlorine" |
Electrochemistry
Electricity. Chemistry. Nothing in common, have nothing to do with each other. Wrong! Electrochemistry has been a fact since 1774. Once electricity was applied to particular solutions, changes occurred that scientists of the time did not expect. In this lab, we will discover some of the same things that Farraday found over 300 years ago. … Continue reading "Electrochemistry" |
Ammonia Experiments
Ammonia has been used by doctors, farmers, chemists, alchemists, weightlifters, and our families since Roman times. Doctors revive unconscious patients, farmers use it in fertilizer, alchemists tried to use it to make gold, weightlifters sniff it into their lungs to invigorate their respiratory system and clear their heads prior to lifting tremendous loads. At home, … Continue reading "Ammonia Experiments" |
Energy from Sugar
This experiment is for advanced students. Purple and white colors, making the whitewash that Tom Sawyer used, and produce an exothermic chemical reaction…..does it get any better? Limewater is one of the compounds we work with in this experiment. Limewater was used in the old days of America. We’re talking about the 80’s…..the 1880’s. Traveling … Continue reading "Energy from Sugar" |
Getting Air from Water
This experiment is for advanced students. This is a repeat of the experiment: Can Fish Drown? but now we’re going to do this experiment again with your new chemistry glassware. The aquarium looked normal in every way, except for the fish. They were breathing very fast and sinking head first to the bottom of the … Continue reading "Getting Air from Water" |
Working with Cataylsts
This experiment is for advanced students. Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done … Continue reading "Working with Cataylsts" |
Hydrogen Peroxide
This experiment is for advanced students. In industry, hydrogen peroxide is used in paper making to bleach the pulp before they form it into paper. Biologists, when preparing bones for display, use peroxide to whiten the bones. At home, 3% peroxide combined with ammonium hydroxide is used to give dark-haired people their desired blonde moment. … Continue reading "Hydrogen Peroxide" |
Generating Oxygen
This experiment is for advanced students. This time we’re going to use a lot of equipment… really break out all the chemistry stuff. We’ll need all this stuff to generate oxygen with potassium permanganate (KMNO4). We will work with this toxic chemical and we will be careful…won’t we? [am4show have=’p9;p52;’ guest_error=’Guest error message’ user_error=’User error … Continue reading "Generating Oxygen" |
Detonating Bubbles
This experiment is for advanced students. Zinc (Zn), is a metal and it is found as element #30 on the periodic table. We need a little zinc to keep our bodies balanced, but too much is very dangerous. Zinc is just like the common, everyday substance that we all know as di-hydrogen monoxide (which is … Continue reading "Detonating Bubbles" |
Desalination
This experiment is for advanced students. Lewis and Clark did this same experiment when they reached the Oregon coast in 1805. Men from the expedition traveled fifteen miles south of the fort they had built at the mouth of the Columbia River to where Seaside, Oregon now thrives. In 1805, however, it was just men … Continue reading "Desalination" |
Carbon Dioxide
This experiment is for advanced students. This lab builds on concepts from the previous carbon dioxide lab. Limewater….carbon dioxide…indicators. We don’t know too much about these things. Sure, we know a little. Carbon dioxide is exhaled by us and plants need it to grow. Burning fossil fuels produces carbon dioxide. Indicators…something we observe that confirms … Continue reading "Carbon Dioxide" |
Zinc Dust
This experiment is for advanced students. Who gets to burn something today? YOU get to burn something today! You will be working with Zinc (Zn). Other labs in this kit allow us to burn metal, but there is a bit of a twist this time. We will be burning a powder. Why a powder instead … Continue reading "Zinc Dust" |
Burning Sulfur
This experiment is for advanced students. Brimstone is another name for sulfur, and if you’ve ever smelled it burn…..whoa….I’m telling you ….you will see for yourself in this lab. It is quite a smell, for sure. Sulfur is element #6 on the periodic table. Sulfur is used in fertilizer, black powder, matches, and insecticides. In … Continue reading "Burning Sulfur" |
Acids and Bases
This experiment is for advanced students. ACID!!! The word causes fear to creep in and get our attention. BASIC!!! The word causes nothing to stir in most of us. The truth is, a strong acid (pH 0-1) is dangerous, but a strong basic (pH 13-14) is just as dangerous. In this lab, we will get … Continue reading "Acids and Bases" |
Making Sodium Hydroxide
This experiment is for advanced students. Ever use soap? Sodium hydroxide (NaOH) is the main component in lye soap. NaOH is mixed with some type of fat (vegetable, pig, cow, etc). Scent can be added for the ‘pretty’ factor and pumice or sand can be added for the manly “You’re coming off my hands and … Continue reading "Making Sodium Hydroxide" |
Potassium Permanganate
This experiment is for advanced students. Potassium permanganate (KMnO4) in water turns an intense, deep, purple. It is important in the film industry for aging props and clothing to make them look much older than they are. Also, artists use it in bone carving. People who carve antlers and bone use KMnO4 to darken the … Continue reading "Potassium Permanganate" |
Potassium Hexacynoferrate (Reagant)
This experiment is for advanced students. How do you know if your brother is stealing your candy? Unwrap a wrapped hard candy that he likes a lot. Roll the candy around in the powdered food dye that matches the candy. (Push the powder into the candy so it “disappears”.) Re-wrap the candy. Set the candy … Continue reading "Potassium Hexacynoferrate (Reagant)" |
Iodine
This experiment is for advanced students. In gas form, element #59 is deadly. However, when iodine is in liquid form, it helps heal cuts and scrapes. The iodine molecule occurs in pairs, not as a single atom (many halogens do this, and it's called a diatomic molecule). It's hard to find iodine in nature, though … Continue reading "Iodine" |
How to Get Hydrogen from Zinc
This experiment is for advanced students. Zinc and Hydrogen are important elements for all of us. Zinc (Zn) metal is element #30 on the periodic table. Lack of zinc in our diets will delay growth of our bodies and can kill. Hydrogen gas (H) is element #1 on the periodic table. Hydrogen was discovered in … Continue reading "How to Get Hydrogen from Zinc" |
Hydrogen Bromide
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students. We've created a video that shows you how to safely do this experiment, although if you're nervous about doing this one, just watch the video and skip the actual experiment. Bromine is a particularly nasty chemical, so be sure to very … Continue reading "Hydrogen Bromide" |
Hydrogen Chlorine Gas
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students. We’ve created a video that shows you how to safely do this experiment, although if you’re nervous about doing this one, just watch the video and skip the actual experiment. The gas you generate with this experiment is lethal in large … Continue reading "Hydrogen Chlorine Gas" |
Hydrogen Peroxide
This experiment is for advanced students. In industry, hydrogen peroxide is used in paper making to bleach the pulp before they form it into paper. Biologists, when preparing bones for display, use peroxide to whiten the bones. At home, 3% peroxide combined with ammonium hydroxide is used to give dark-haired people their desired blonde moment. … Continue reading "Hydrogen Peroxide" |