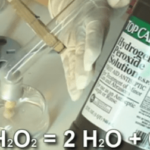Special Science Teleclass: Chemistry & Chemical Engineering
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I've included it here so you can participate and learn, too! We’re going to be mixing up dinosaur toothpaste, doing experiments with catalysts, discovering the 5 states of matter, and building your own chemistry lab … Continue reading "Special Science Teleclass: Chemistry & Chemical Engineering" |
Bicarbonate Buffer
This experiment is for advanced students. All chemical reactions are equilibrium reactions. This experiment is really cool because you’re going to watch how a chemical reaction resists a pH change. |
Hydrolysis
This experiment is for advanced students. Hydrolysis is a chemical reaction that involves breaking a molecular bond using water. In chemistry, there are three different types of hydrolysis: sat hydrolysis, acid hydrolysis, and base hydrolysis. In nature, living organisms survive by making their energy from processing food. The energy converted from food is stored in ATP … Continue reading "Hydrolysis" |
Redox Reaction
This experiment is for advanced students. We’re going to look at the strength of redox reactions using copper, zinc, and acids. |
Balancing Chemical Equations
Chemistry is all about studying chemical reactions and the combinations of elements and molecules that combine to give new stuff. Chemical reactions can be written down as a balanced equation that shows how much of each molecule and compound are needed for that particular reaction. Here’s how you do it: |
Temperature and Le Chatelier’s Principle
If you’re into magic shows, this is a good one to perform for an audience, because the solution goes from purple to pink to green to blue and back again! Le Chatelier’s principle states that when the temperature is raised, an equilibrium will shift away from the side that contains energy. When temperature is lowered, … Continue reading "Temperature and Le Chatelier’s Principle" |
Effect of Temperature on Solubility
We’re going to do an experiment where it will look like we can boil soda on command… but the truth is, it’s not really boiling in the first place! If you drink soda, save one for doing this experiment. Otherwise, get one that’s “diet” (without the sugar, it’s a lot easier to clean up). |
Measuring the Size of a Molecule
Molecules are the building blocks of matter. You’ve probably heard that before, right? But that does it mean? What does a molecule look like? How big are they? While you technically can measure the size of a molecule, despite the fact it’s usually too small to do even with a regular microscope, what you can’t … Continue reading "Measuring the Size of a Molecule" |
Basic Chemistry Safety Information
Chemical Data & Safe Handling Information Sheet What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW! If you haven’t … Continue reading "Basic Chemistry Safety Information" |
Making Litmus Solution and Paper
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH. Every liquid has a pH. If you pay particular attention to this lab, you will … Continue reading "Making Litmus Solution and Paper" |
Energy from Sugar
This experiment is for advanced students. Purple and white colors, making the whitewash that Tom Sawyer used, and produce an exothermic chemical reaction…..does it get any better? Limewater is one of the compounds we work with in this experiment. Limewater was used in the old days of America. We’re talking about the 80’s…..the 1880’s. Traveling … Continue reading "Energy from Sugar" |
Getting Air from Water
This experiment is for advanced students. This is a repeat of the experiment: Can Fish Drown? but now we’re going to do this experiment again with your new chemistry glassware. The aquarium looked normal in every way, except for the fish. They were breathing very fast and sinking head first to the bottom of the … Continue reading "Getting Air from Water" |
Working with Cataylsts
This experiment is for advanced students. Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done … Continue reading "Working with Cataylsts" |
Hydrogen Peroxide
This experiment is for advanced students. In industry, hydrogen peroxide is used in paper making to bleach the pulp before they form it into paper. Biologists, when preparing bones for display, use peroxide to whiten the bones. At home, 3% peroxide combined with ammonium hydroxide is used to give dark-haired people their desired blonde moment. … Continue reading "Hydrogen Peroxide" |
Generating Oxygen
This experiment is for advanced students. This time we’re going to use a lot of equipment… really break out all the chemistry stuff. We’ll need all this stuff to generate oxygen with potassium permanganate (KMNO4). We will work with this toxic chemical and we will be careful…won’t we? |
Detonating Bubbles
This experiment is for advanced students. Zinc (Zn), is a metal and it is found as element #30 on the periodic table. We need a little zinc to keep our bodies balanced, but too much is very dangerous. Zinc is just like the common, everyday substance that we all know as di-hydrogen monoxide (which is … Continue reading "Detonating Bubbles" |
Desalination
This experiment is for advanced students. Lewis and Clark did this same experiment when they reached the Oregon coast in 1805. Men from the expedition traveled fifteen miles south of the fort they had built at the mouth of the Columbia River to where Seaside, Oregon now thrives. In 1805, however, it was just men … Continue reading "Desalination" |
Cold Light
This experiment is for advanced students. Glo-sticks! Parents hang them from their trick or treaters, backpackers read with them light late at night in a tent. Glo-sticks work on the principle of chemiluminescence. Chemiluminescence is defined as emitting light without heat as the result of a chemical reaction. |
Carbon Dioxide
This experiment is for advanced students. This lab builds on concepts from the previous carbon dioxide lab. Limewater….carbon dioxide…indicators. We don’t know too much about these things. Sure, we know a little. Carbon dioxide is exhaled by us and plants need it to grow. Burning fossil fuels produces carbon dioxide. Indicators…something we observe that confirms … Continue reading "Carbon Dioxide" |
Zinc Dust
This experiment is for advanced students. Who gets to burn something today? YOU get to burn something today! You will be working with Zinc (Zn). Other labs in this kit allow us to burn metal, but there is a bit of a twist this time. We will be burning a powder. Why a powder instead … Continue reading "Zinc Dust" |
Magnesium Battery
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum … Continue reading "Magnesium Battery" |
Burning Sulfur
This experiment is for advanced students. Brimstone is another name for sulfur, and if you’ve ever smelled it burn…..whoa….I’m telling you ….you will see for yourself in this lab. It is quite a smell, for sure. Sulfur is element #6 on the periodic table. Sulfur is used in fertilizer, black powder, matches, and insecticides. In … Continue reading "Burning Sulfur" |
Making Copper
In this lab, we’re going to investigate the wonders of electrochemistry. Electrochemistry became a new branch of chemistry in 1832, founded by Michael Faraday. Michael Faraday is considered the “father of electrochemistry”. The knowledge gained from his work has filtered down to this lab. YOU will be like Michael Faraday. I imagined he would have … Continue reading "Making Copper" |
Making Chlorine
If we don’t have salt, we die. It’s that simple. The chemical formula for salt is NaCl. Broken down, we have Na (sodium) and Cl (chlorine). Either one of these can be fatal in sufficient quantities. When chemically combined, these two deadly elements become table salt. What once could kill now keeps us alive. Isn’t … Continue reading "Making Chlorine" |
Electrochemistry
Electricity. Chemistry. Nothing in common, have nothing to do with each other. Wrong! Electrochemistry has been a fact since 1774. Once electricity was applied to particular solutions, changes occurred that scientists of the time did not expect. In this lab, we will discover some of the same things that Farraday found over 300 years ago. … Continue reading "Electrochemistry" |
Ammonia Experiments
Ammonia has been used by doctors, farmers, chemists, alchemists, weightlifters, and our families since Roman times. Doctors revive unconscious patients, farmers use it in fertilizer, alchemists tried to use it to make gold, weightlifters sniff it into their lungs to invigorate their respiratory system and clear their heads prior to lifting tremendous loads. At home, … Continue reading "Ammonia Experiments" |
Acids and Bases
This experiment is for advanced students. ACID!!! The word causes fear to creep in and get our attention. BASIC!!! The word causes nothing to stir in most of us. The truth is, a strong acid (pH 0-1) is dangerous, but a strong basic (pH 13-14) is just as dangerous. In this lab, we will get … Continue reading "Acids and Bases" |
Making Sodium Hydroxide
This experiment is for advanced students. Ever use soap? Sodium hydroxide (NaOH) is the main component in lye soap. NaOH is mixed with some type of fat (vegetable, pig, cow, etc). Scent can be added for the ‘pretty’ factor and pumice or sand can be added for the manly “You’re coming off my hands and … Continue reading "Making Sodium Hydroxide" |
Potassium Permanganate
This experiment is for advanced students. Potassium permanganate (KMnO4) in water turns an intense, deep, purple. It is important in the film industry for aging props and clothing to make them look much older than they are. Also, artists use it in bone carving. People who carve antlers and bone use KMnO4 to darken the … Continue reading "Potassium Permanganate" |
Potassium Hexacynoferrate (Reagant)
This experiment is for advanced students. How do you know if your brother is stealing your candy? Unwrap a wrapped hard candy that he likes a lot. Roll the candy around in the powdered food dye that matches the candy. (Push the powder into the candy so it “disappears”.) Re-wrap the candy. Set the candy … Continue reading "Potassium Hexacynoferrate (Reagant)" |
Iron Sparklers
This experiment is for advanced students. Sparks flying off in all directions…that’s fun. In this lab, we will show how easy it is to produce those shooting sparks. In a sparkler you buy at the store, the filings used are either iron or aluminum. The filings are placed in a mixture that, when dry, adheres … Continue reading "Iron Sparklers" |
Iodine
This experiment is for advanced students. In gas form, element #59 is deadly. However, when iodine is in liquid form, it helps heal cuts and scrapes. The iodine molecule occurs in pairs, not as a single atom (many halogens do this, and it’s called a diatomic molecule). It’s hard to find iodine in nature, though … Continue reading "Iodine" |
How to Get Hydrogen from Zinc
This experiment is for advanced students. Zinc and Hydrogen are important elements for all of us. Zinc (Zn) metal is element #30 on the periodic table. Lack of zinc in our diets will delay growth of our bodies and can kill. Hydrogen gas (H) is element #1 on the periodic table. Hydrogen was discovered in … Continue reading "How to Get Hydrogen from Zinc" |
Hydrogen Bromide
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students. We’ve created a video that shows you how to safely do this experiment, although if you’re nervous about doing this one, just watch the video and skip the actual experiment. Bromine is a particularly nasty chemical, so be sure to very … Continue reading "Hydrogen Bromide" |
Hydrogen Chlorine Gas
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students. We’ve created a video that shows you how to safely do this experiment, although if you’re nervous about doing this one, just watch the video and skip the actual experiment. The gas you generate with this experiment is lethal in large … Continue reading "Hydrogen Chlorine Gas" |
Colored Campfires and Rainbows
Always have a FIRE EXTINGUISHER and ADULT HELP handy when performing fire experiments. NO EXCEPTIONS. This video will show you how to transform the color of your flames. For a campfire, simply sprinkle the solids into your flames (make sure they are ground into a fine powder first) and you’ll see a color change. DO … Continue reading "Colored Campfires and Rainbows" |
Calibrated Spectrometer
Ever play with a prism? When sunlight strikes the prism, it gets split into a rainbow of colors. Prisms un-mix the light into its different wavelengths (which you see as different colors). Diffraction gratings are tiny prisms stacked together.When light passes through a diffraction grating, it splits (diffracts) the light into several beams traveling at … Continue reading "Calibrated Spectrometer" |
Going Further with Advanced Chemistry
This experiment is for advanced students. One of my best teaching tools for science developed from a brain freeze one afternoon in class. I went to the board to draw the chlorophyll wheel and drew a complete blank. “Let’s say I forgot how to draw the wheel.” I turned to the class, marker in hand, … Continue reading "Going Further with Advanced Chemistry" |
Electrolysis
If you guessed that this has to do with electricity and chemistry, you’re right! But you might wonder how they work together. Back in 1800, William Nicholson and Johann Ritter were the first ones to split water into hydrogen and oxygen using electrolysis. |