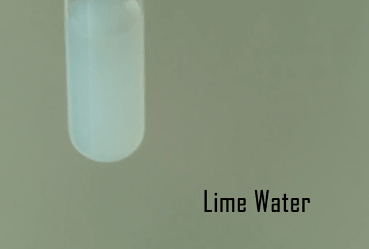Calcium is an element that is softer than aluminum but harder than sodium, and it’s less chemically reactive than alkaline metals. Have you ever looked inside of a water pipe? The hard, white crust you see is probably calcium or magnesium deposits (especially if you have “hard” water). When calcium comes in contact with air, it forms an oxide coating which protects it from further corrosion.
Please login or register to read the rest of this content.
Tannins are everywhere in nature: in wood, bark, leaves, and fruit of plants like walnut, cranberry, cacao, grapes, and oak trees. Tannins are how plants make themselves unappetizing to animals, so they won’t eat the fruit before it’s ripe. Have you ever eaten an unripe pear or plum? That’s the tannin you’re tasting! People have used tannins to “tan” animal hides to make leather. People like tannins in their food, because it can be managed to make the food taste really good, like in chocolate, tea, coffee, and wine.
Please login or register to read the rest of this content.
We’re going to look at the properties of sugar. We’re going to combine sugar in different solutions to explore the interesting properties it has, including how even though sugar is sweet, something like apple juice, which has a lot of sugar in it, is actually acidic! It’s important that you DO NOT EAT ANYTHING in this lab. This is a CHEMISTRY LAB.
Please login or register to read the rest of this content.
Surfactants are compounds that decrease the surface tension between two liquids, a liquid and a gas, or a liquid and a solid. You’ll find surfactants acting as detergents (in laundry soaps), emulsifiers (like egg yolks and soy lecithin), foaming agents (like in foaming soaps, shampoos, and toothpaste), and dispersants (usually added to prevent clumping, like in engine oils and in concretes so it flows correctly before setting). Not all surfactants are the same, but they all are aimed at doing a particular job.
Please login or register to read the rest of this content.
We’re going to do experiments on the water molecule to discover more about water! We’ll need the lime water solution from a previous experiment. Water is a chemical compound and a polar molecule, and it’s a liquid at atmospheric temperature and pressure. The solid state is known as “ice” and the gaseous form is “water vapor”.
Please login or register to read the rest of this content.
We’re going to learn about saturated solutions today! A solution with solute (the powder added in) that dissolves until it leaves bits at the bottom (indicating that it cannot dissolve anymore). An unsaturated solution is the solution before the bits start showing up at the bottom, meaning that there’s enough solvent to hold all the solute you add in.
Please login or register to read the rest of this content.
Sodium chloride, ammonium chloride, sodium carbonate, and many others have tiny charged particles (positive and negative) called “salts”. When a “salt” is dissolved in water, it will separate into the two particles (plus and minus), which means that if you pass a current through the solution, the positive particles (positive ions) become attracted to the negative pole and the negative particles (negative ions) move toward the positive pole. This movement is allowing electricity to flow, and the this is the reason that “salt” solutions conduct electricity.
Please login or register to read the rest of this content.
We’re using the solution from the last experiment (the iron in what used to be a copper sulfate solution) for part of this experiment. This experiment is tricky to see a color change, which is why we’re going to look at the paper towel for the telltale blue that will indicate the presence of iron.
Please login or register to read the rest of this content.
We’re going to look at how iron reacts with an acid and detect the iron bits with an indicator. We’re going to do several experiments that will need a bit more time, like overnight, so be sure to store this experiment out of reach of small kids while you are waiting for it to progress.
Please login or register to read the rest of this content.
We’re going to learn about the properties of combustion by doing a simple set of experiments. Because this involves FIRE, please make sure you have an adult handy with you while you do your experiments. We’re going to learn how to detect the presence of carbon dioxide by looking for a “precipitate” – tiny little particles that seem to form out of nowhere.
Please login or register to read the rest of this content.
This is a neat set of experiments, and the trick works because carbon dioxide is heavier than air so it really can be “poured” just like a liquid. The problem is that most people mis-judge the “pour”, so if you want to practice first, capture the smoke from an extinguished candle first (get an adult to help you!) and practice pouring a gas into a bowl.
Please login or register to read the rest of this content.
This experiment is in two parts. We’re going to use chemistry to separate mixtures. We’re going to use a mixture we prepared in the previous experiment. Also, please make sure you displose of the copper sulfate correctly, you can’t simply just throw it in the trash or down the drain.
Please login or register to read the rest of this content.
Water is measured in inches or centimetres when it’s in a test tube. We’re going to make a solution that we will be keeping for not only today’s but also future experiments as well. The solution is hazardous to aquatic life, so make sure you watch all the disposal instructions near the end of the video.
You’re going to create a beautiful blue color by combining two substances together, one is an indicator for the other (the iron).
Please login or register to read the rest of this content.
You’ll find carbon dioxide everywhere: when you exhale, the bubbles in your soda, in Venus’ atmosphere… The next set of experiments bounce around a little within the manual that came with your set of chemicals. We put all of them together here because it makes the most sense – watch and you’ll see!
It’s hard to know what molecules are present in a solution, so with litmus and limewater, you now have ways to detect these! This is only the start… let’s watch the video now to learn more.
Please login or register to read the rest of this content.
Litmus is from a plant, so it will have a limited shelf life (you’ll notice a different, more earthy smell to it). The amount of dry powder provided in the kit is enough for three solutions, more than enough for our experiments. If you notice particles and sludge at the bottom of your container, it’s totally normal, and all you need to do is pour the liquid off and scrape out the residue and throw (the residue) away. If you add a little denatured alcohol (ethyl alcohol), the solution will last a bit longer on the shelf.
We’re going to do a number of experiments with this solution, all in one video. My friend, a PhD Chemistry professor, helped make a new set of videos for you that will walk you through every step.
Please login or register to read the rest of this content.
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too! (Click here if you’re looking for the more recent version that also includes Chemical Engineering.)
When you think of slime, do you imagine slugs, snails, and puppy kisses? Or does the science fiction film The Blob come to mind? Any way you picture it, slime is definitely slippery, slithery, and just plain icky — and a perfect forum for learning real science. But which ingredients work in making a truly slimy concoction, and why do they work? Let’s take a closer look…
Materials:
- Sodium tetraborate (also called “Borax” – it’s a laundry whitener) – about 2 tablespoons
- Clear glue or white glue (clear works better if you can find it) – about 1/2 cup
- Yellow highlighter
- Pliers or sharp razor (with adult help). (PREPARE: Use this to get the end off your highlighter before class starts so you can extract the ink-soaked felt inside. Leave the felt inside highlighter with the end loosely on (so it doesn’t dry out))
- Resuable Instant Hand Warmer that contains sodium acetate (Brand Name: EZ Hand Warmer) – you’ll need two of these
- Scissors
- Glass half full of COLD water (PREPARE: put this in the fridge overnight)
- Mixing bowl full of ice (PREPARE: leave in freezer)
- Salt
- Disposable aluminum pie place or foil-wrapped paper plate
- Disposable cups for solutions (4-6)
- Popsicle sticks for mixing (4-6)
- Rubber gloves for your hands
- Optional: If you want to see your experiments glow in the dark, you’ll need a fluorescent UV black light (about $10 from the pet store – look in cleaning supplies under “Urine-Off” for a fluorescent UV light). UV flashlights and UV LEDs will not work.
Chemistry is all about studying chemical reactions and the combinations of elements and molecules that combine to give new stuff. Chemical reactions can be written down as a balanced equation that shows how much of each molecule and compound are needed for that particular reaction. Here’s how you do it:
Please login or register to read the rest of this content.
Chemical Data & Safe Handling Information Sheet
What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW!
When you’re done storing your chemicals out of reach, watch this video:
Please login or register to read the rest of this content.
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH.
Every liquid has a pH. If you pay particular attention to this lab, you will even be able to identify most acids and bases and understand why they do what they do. Acids range from very strong to very weak. The strongest acids will dissolve steel. The weakest acids are in your drink box. The strongest bases behave similarly. They can burn your skin or you can wash your hands with them.
Acid rain is one aspect of low pH that you can see every day if you look for it. This is a strange name, isn’t it? We get rained on all the time. If people were dissolving, if the rain made their skin smoke and burn, you’d think it would make headlines, wouldn’t you? The truth is acid rain is too weak to harm us except in very rare and localized conditions. But it’s hard on limestone buildings.
Please login or register to read the rest of this content.
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum to create the BMW N52 6-cylinder magnesium engine block. Photographers used to use magnesium powder in the camera’s flash before xenon bulbs were available.
Most folks, however, equate magnesium with a burning white flame. Magnesium fires burn too hot to be extinguished using water, so most firefighters use sand or graphite.
We’re going to learn how to (safely) ignite a piece of magnesium in the first experiment, and next how to get energy from it by using it in a battery in the second experiment. Are you ready?
Please login or register to read the rest of this content.
In this lab, we’re going to investigate the wonders of electrochemistry. Electrochemistry became a new branch of chemistry in 1832, founded by Michael Faraday. Michael Faraday is considered the “father of electrochemistry”. The knowledge gained from his work has filtered down to this lab. YOU will be like Michael Faraday. I imagined he would have been overjoyed to do this lab and see the results. You are soooo lucky to be able to take an active part in this experiment. Here’s what you’re going to do…
You will be “creating” metallic copper from a solution of copper sulfate and water, and depositing it on a negative electrode. Copper is one of our more interesting elements. Copper is a metal, and element 29 on your periodic table. It conducts heat and electricity very well.
Many things around you are made of copper. Copper wire is used in electrical wiring. It has been used for centuries in the form of pipes to distribute water and other fluids in homes and in industry. The Statue of Liberty is a wonderful example of how beautiful 180,000 pounds of copper can be. Yes, it is made of copper, and no, it doesn’t look like a penny…..on the surface. The green color is copper oxide, which forms on the surface of copper exposed to air and water. The oxide is formed on the surface and does not attack the bulk of the copper. You could say that copper oxide protects the copper.
Please login or register to read the rest of this content.
If we don’t have salt, we die. It’s that simple. The chemical formula for salt is NaCl. Broken down, we have Na (sodium) and Cl (chlorine). Either one of these can be fatal in sufficient quantities. When chemically combined, these two deadly elements become table salt. What once could kill now keeps us alive. Isn’t chemistry awesome?
Chlorine, element 17, is called a halogen as are all the elements in the 17th row. All halogens have similar chemical properties. They are highly reactive nonmetals, and react easily with most metals. Sodium is a metal, and is bonded with sodium in the table salt used in this lab. Besides being found in salt, chlorine has many uses in our world such as killing bacteria in our water, making plastic, cleaning products, and the list goes on. A very useful chemical, and is among the top ten chemicals produced in the United States. Ever since its discovery in 1774, chlorine has been very useful. It is found in nature in sodium chloride, but in very small concentrations. Seawater, the most abundant source of chlorine, has a concentration of only 19g of chlorine per liter.
Please login or register to read the rest of this content.
Electricity. Chemistry. Nothing in common, have nothing to do with each other. Wrong! Electrochemistry has been a fact since 1774. Once electricity was applied to particular solutions, changes occurred that scientists of the time did not expect.
In this lab, we will discover some of the same things that Farraday found over 300 years ago. We will be there as things tear apart, particles rush about, and the power of attraction is very strong. We’re not talking about dancing, we’re talking about something much more important and interesting….we’re talking about ELECTROCHEMISTRY!
Please login or register to read the rest of this content.
Ammonia has been used by doctors, farmers, chemists, alchemists, weightlifters, and our families since Roman times. Doctors revive unconscious patients, farmers use it in fertilizer, alchemists tried to use it to make gold, weightlifters sniff it into their lungs to invigorate their respiratory system and clear their heads prior to lifting tremendous loads. At home, ammonia is used to clean up the ketchup you spilled on the floor and never cleaned up.
The ammonia molecule (NH3) is a colorless gas with a strong odor – it’s the smell of freshly cleaned floors and windows. Mom is not cleaning with straight ammonia (it’s gas at room temperature because it boils at -28oF, so the stuff she cleans with is actually ammonium hydroxide, a solution of ammonia and water). Ammonia is found when plants and animals decompose, and it’s also in rainwater, volcanoes, your kidneys (to neutralize excess acid), in the ocean, some fertilizers, in Jupiter’s lower cloud decks, and trace amounts are found in our own atmosphere (it’s lighter than air).
Please login or register to read the rest of this content.
Chemical Data & Safe Handling Information Sheet
What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW!
If you haven’t already done so, make sure to watch the introductory video for the Intermediate Chemistry and Advanced Chemistry lessons. They contain important information about the chemicals and lab equipment you’ll be using. When you’re done storing your chemicals out of reach, watch this video:
Please login or register to read the rest of this content.
Let’s see how much you’ve picked up with these experiments and the reading – answer as best as you can. (No peeking at the answers until you’re done!) Just relax and see what jumps to mind when you read the question. You can also print these out and jot down your answers in your science notebook.
Please login or register to read the rest of this content.
Let’s see how you did! If you didn’t get a few of these, don’t let it stress you out – it just means you need to play with more experiments in this area. We’re all works in progress, and we have our entire lifetime to puzzle together the mysteries of the universe!
Here’s printer-friendly versions of the exercises and answers for you to print out: Simply click here for printable questions and answers.
Answers:
Please login or register to read the rest of this content.
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH.
Every liquid has a pH. If you pay particular attention to this lab, you will even be able to identify most acids and bases and understand why they do what they do. Acids range from very strong to very weak. The strongest acids will dissolve steel. The weakest acids are in your drink box. The strongest bases behave similarly. They can burn your skin or you can wash your hands with them.
Acid rain is one aspect of low pH that you can see every day if you look for it. This is a strange name, isn’t it? We get rained on all the time. If people were dissolving, if the rain made their skin smoke and burn, you’d think it would make headlines, wouldn’t you? The truth is acid rain is too weak to harm us except in very rare and localized conditions. But it’s hard on limestone buildings.
Please login or register to read the rest of this content.
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum to create the BMW N52 6-cylinder magnesium engine block. Photographers used to use magnesium powder in the camera’s flash before xenon bulbs were available.
Most folks, however, equate magnesium with a burning white flame. Magnesium fires burn too hot to be extinguished using water, so most firefighters use sand or graphite.
We’re going to learn how to (safely) ignite a piece of magnesium in the first experiment, and next how to get energy from it by using it in a battery in the second experiment. Are you ready?
Please login or register to read the rest of this content.
In this lab, we’re going to investigate the wonders of electrochemistry. Electrochemistry became a new branch of chemistry in 1832, founded by Michael Faraday. Michael Faraday is considered the “father of electrochemistry”. The knowledge gained from his work has filtered down to this lab. YOU will be like Michael Faraday. I imagined he would have been overjoyed to do this lab and see the results. You are soooo lucky to be able to take an active part in this experiment. Here’s what you’re going to do…
You will be “creating” metallic copper from a solution of copper sulfate and water, and depositing it on a negative electrode. Copper is one of our more interesting elements. Copper is a metal, and element 29 on your periodic table. It conducts heat and electricity very well.
Many things around you are made of copper. Copper wire is used in electrical wiring. It has been used for centuries in the form of pipes to distribute water and other fluids in homes and in industry. The Statue of Liberty is a wonderful example of how beautiful 180,000 pounds of copper can be. Yes, it is made of copper, and no, it doesn’t look like a penny…..on the surface. The green color is copper oxide, which forms on the surface of copper exposed to air and water. The oxide is formed on the surface and does not attack the bulk of the copper. You could say that copper oxide protects the copper.
Please login or register to read the rest of this content.
If we don’t have salt, we die. It’s that simple. The chemical formula for salt is NaCl. Broken down, we have Na (sodium) and Cl (chlorine). Either one of these can be fatal in sufficient quantities. When chemically combined, these two deadly elements become table salt. What once could kill now keeps us alive. Isn’t chemistry awesome?
Chlorine, element 17, is called a halogen as are all the elements in the 17th row. All halogens have similar chemical properties. They are highly reactive nonmetals, and react easily with most metals. Sodium is a metal, and is bonded with sodium in the table salt used in this lab. Besides being found in salt, chlorine has many uses in our world such as killing bacteria in our water, making plastic, cleaning products, and the list goes on. A very useful chemical, and is among the top ten chemicals produced in the United States. Ever since its discovery in 1774, chlorine has been very useful. It is found in nature in sodium chloride, but in very small concentrations. Seawater, the most abundant source of chlorine, has a concentration of only 19g of chlorine per liter.
Please login or register to read the rest of this content.
Electricity. Chemistry. Nothing in common, have nothing to do with each other. Wrong! Electrochemistry has been a fact since 1774. Once electricity was applied to particular solutions, changes occurred that scientists of the time did not expect.
In this lab, we will discover some of the same things that Farraday found over 300 years ago. We will be there as things tear apart, particles rush about, and the power of attraction is very strong. We’re not talking about dancing, we’re talking about something much more important and interesting….we’re talking about ELECTROCHEMISTRY!
Please login or register to read the rest of this content.
Ammonia has been used by doctors, farmers, chemists, alchemists, weightlifters, and our families since Roman times. Doctors revive unconscious patients, farmers use it in fertilizer, alchemists tried to use it to make gold, weightlifters sniff it into their lungs to invigorate their respiratory system and clear their heads prior to lifting tremendous loads. At home, ammonia is used to clean up the ketchup you spilled on the floor and never cleaned up.
The ammonia molecule (NH3) is a colorless gas with a strong odor – it’s the smell of freshly cleaned floors and windows. Mom is not cleaning with straight ammonia (it’s gas at room temperature because it boils at -28oF, so the stuff she cleans with is actually ammonium hydroxide, a solution of ammonia and water). Ammonia is found when plans and animals decompose, and it’s also in rainwater, volcanoes, your kidneys (to neutralize excess acid), in the ocean, some fertilizers, in Jupiter’s lower cloud decks, and trace amounts are found in our own atmosphere (it’s lighter than air).
Please login or register to read the rest of this content.
In this unit, you will learn how to build your own home chemistry lab safely under the direction of professionals. We’ll show you how to do real chemistry experiments, provide chemical storage information, give guidelines on proper chemical disposal when you’re finished, highlight lab tips and tricks, and warn you about things to watch out for. This is real chemistry for real kids.
Please login or register to read the rest of this content.
This experiment is just for advanced students. If you guessed that this has to do with electricity and chemistry, you’re right! But you might wonder how they work together. Back in 1800, William Nicholson and Johann Ritter were the first ones to split water into hydrogen and oxygen using electrolysis. (Soon afterward, Ritter went on to figure out electroplating.) They added energy in the form of an electric current into a cup of water and captured the bubbles forming into two separate cups, one for hydrogen and other for oxygen.
This experiment is not an easy one, so feel free to skip it if you need to. You don’t need to do this to get the concepts of this lesson but it’s such a neat and classical experiment (my students love it) so you can give it a try if you want to. The reason I like this is because what you are really doing in this experiment is ripping molecules apart and then later crashing them back together.
Have fun and please follow the directions carefully. This could be dangerous if you’re not careful. The image shown here is using graphite from two pencils sharpened on both ends, but the instructions below use wire. Feel free to try both to see which types of electrodes provide the best results.