 Everyone old enough to remember the Rubik’s Cube craze of the 1980s in the USA also remembers how it was near impossible to solve the thing! Originally created by a professor of architecture Erno Rubik, it was sold to a toy company in 1980 as the “Magic Cube”.
Everyone old enough to remember the Rubik’s Cube craze of the 1980s in the USA also remembers how it was near impossible to solve the thing! Originally created by a professor of architecture Erno Rubik, it was sold to a toy company in 1980 as the “Magic Cube”.
To date, over 350 million cubes have been sold worldwide, making it the world’s top selling puzzle game, and most people think of it as the best-selling toy of all time as well.
The original goal of creating this object was to help teach his students how to create something that rotated independently in layers without falling apart. Rubik didn’t realize he had created a puzzle until he scrambled it, and it took him over a month to solve it the first time!
There are eight corners and twelve edges, and when you do the math to figure out the number of possible combinations the puzzle has, it’s about 43 quintillion, or:
43,252,003,274,489,856,000
So what do you do with this thing? How DO you solve it?
It has to do with identifying the different layers, and solving one layer at a time. Here’s how you can do it:
Please
login or
register to read the rest of this content.
Download the official solver’s guide
here. Or you can build a LEGO machine like JP Brown did to solve it for you!
There’s also a World Cube Association where folks keep track of cube competitions and records. The fastest cube solve was set by Mats Valk in 2013 – he can solve it in under 6 seconds. Some of the more creative competitions include solving the cube while blindfolded (record is 23.8 seconds), with only one hand (record is 12.6 seconds), only using the feet (record is 27.93 seconds), and underwater using a single breath.



 Everyone old enough to remember the Rubik’s Cube craze of the 1980s in the USA also remembers how it was near impossible to solve the thing! Originally created by a professor of architecture Erno Rubik, it was sold to a toy company in 1980 as the “Magic Cube”.
Everyone old enough to remember the Rubik’s Cube craze of the 1980s in the USA also remembers how it was near impossible to solve the thing! Originally created by a professor of architecture Erno Rubik, it was sold to a toy company in 1980 as the “Magic Cube”.



 Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!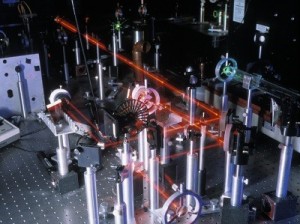 An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).
An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).