 This super-cool project lets kids have the fun of playing tag in the dark on a warm summer evening, without the "gun" aspect traditionally found in laser tag. Kids not only get to enjoy the sport, but also have the pride that they build the tag system themselves - something you simply can't get from opening up a laser tag game box.
This super-cool project lets kids have the fun of playing tag in the dark on a warm summer evening, without the "gun" aspect traditionally found in laser tag. Kids not only get to enjoy the sport, but also have the pride that they build the tag system themselves - something you simply can't get from opening up a laser tag game box.
While real laser tag games actually never use lasers, but rather infrared beams, this laser tag uses real lasers, so you'll want to arm the kids with the "no-lasers-on-the-face" with a 10-minute time-out penalty to ensure everyone has a good time. You can alternatively use flashlights instead of lasers, which makes the game a lot easier to tag someone out.
This game uses a simple two-transistor latching circuit design, so there's no programming or overly-complicated circuitry to worry about. If you've never built this kind of circuit before, it's a perfect first-step into the world of electronics.
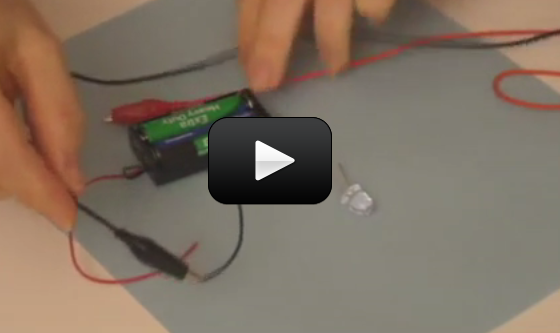
I've provided you with three videos below. This first video is an introduction to what we are going to make and how it works. Here's what you need:
NOTE: We updated this circuit in 2023 to reflect "best practices" when using transistors.
Be sure to build this project as shown in the schematic and breadboard diagrams, and not as shown in the video.
The material list below is based on the new design as shown in the schematic and breadboard diagrams on this page.
The videos show how to build the old circuit, but are still very useful.
Materials (the list below builds one complete set per kid):
- Two AA battery packs with batteries
- LED (any color)
- 51Ω resistor
- 10KΩ resistor
- 1KΩ resistor
- 470Ω resistor
- NPN transistor (2N3904 or 2N2222)
- PNP transistor (2N3906 or 2N4403)
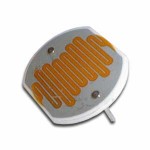
- CdS Cell
- Optional: N/O pushbutton switch
- Breadboard OR soldering equipment (including wire strippers, diagonal cutters, solder...)
- Flashlight or red (NOT green!!) laser
Flashlight Laser Tag Schematic:
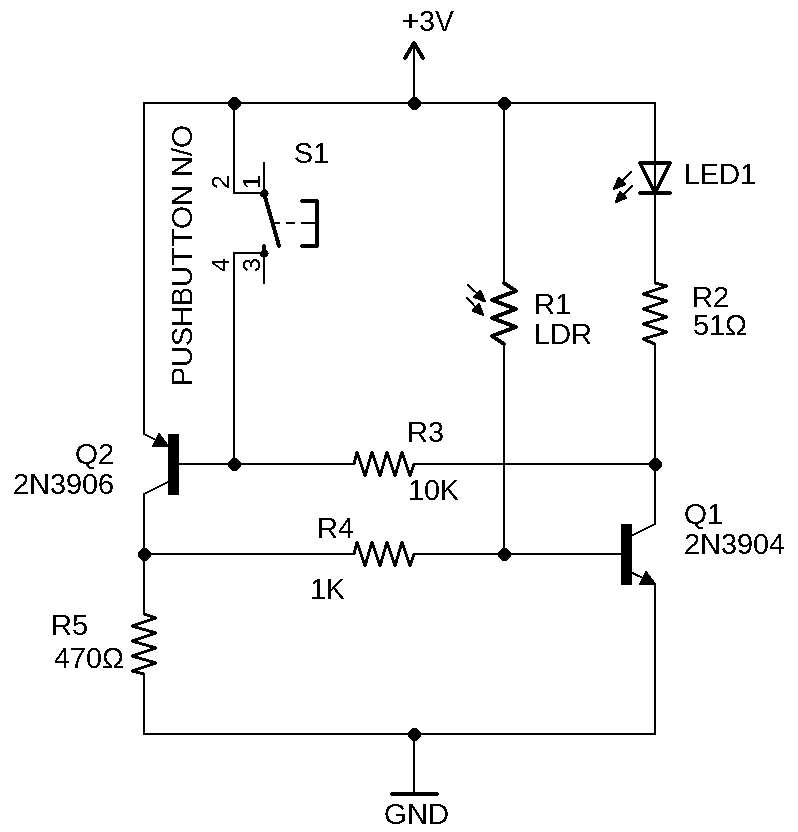
Flashlight laser tag breadboard diagram: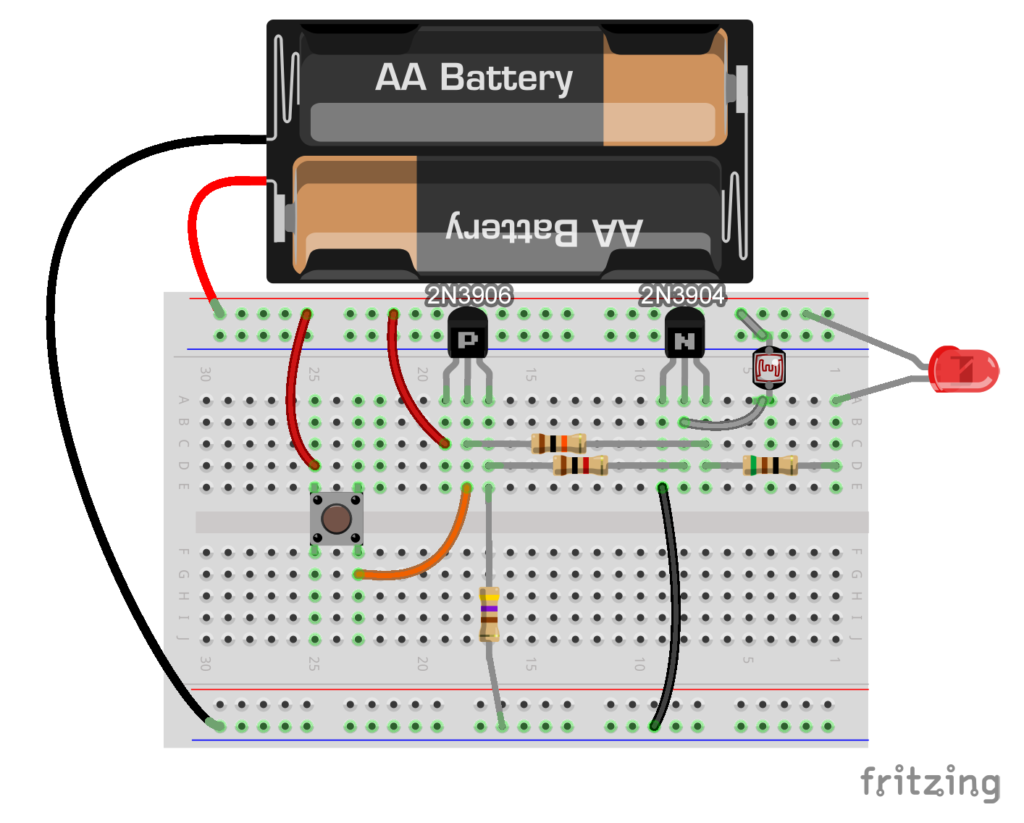
Introduction to the Circuit
The next two videos below show you how to build the circuit, first on a breadboard, and then how to solder the circuit together, so you can opt to watch either one. If you have someone who's handy with tools and soldering irons, invite them to build this with you.
Building the Circuit on a Breadboard
Soldering the Circuit Together
You'll need one of these circuits for every player, although you can get by with one kid having a flashlight (this is the "it" person) and the other running around wearing the circuit trying not to get "tagged". You can mount these circuits inside a soap box or cardboard box with the sensor and light peeking out. Add a belt or wrist strap and you're ready for action!











 Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!





 Imagine you’re a painter. What three colors do you need to make up any color in the universe? (You should be thinking: red, yellow, and blue… and yes, you are right if you’re thinking that the real primary colors are cyan, magenta, and yellow, but some folks still prefer to think of the primary colors as red-yellow-blue… either way, it’s really not important to this experiment which primary set you choose.)
Imagine you’re a painter. What three colors do you need to make up any color in the universe? (You should be thinking: red, yellow, and blue… and yes, you are right if you’re thinking that the real primary colors are cyan, magenta, and yellow, but some folks still prefer to think of the primary colors as red-yellow-blue… either way, it’s really not important to this experiment which primary set you choose.)









 What happens when you shine a laser beam onto a spinning mirror? In the
What happens when you shine a laser beam onto a spinning mirror? In the 
 This experiment is for advanced students.Did you know that when you talk inside a house, the windows vibrate very slightly from your voice? If you stand outside the house and aim a laser beam at the window, you can pick up the vibrations in the window and actually hear the conversation inside the house.
This experiment is for advanced students.Did you know that when you talk inside a house, the windows vibrate very slightly from your voice? If you stand outside the house and aim a laser beam at the window, you can pick up the vibrations in the window and actually hear the conversation inside the house.
 So you’ve played with lenses, mirrors, and built an optical bench. Want to make a real telescope? In this experiment, you’ll build a Newtonian and a refractor telescope using your
So you’ve played with lenses, mirrors, and built an optical bench. Want to make a real telescope? In this experiment, you’ll build a Newtonian and a refractor telescope using your 


 Most resources that public school advisers suggest for gifted or bright kids are a ‘mile wide and an inch deep’ – they don’t really go into depth on any one area. After traveling to dozens of home school conventions for several years across the country and seeing what math options are out there, I searched for more options than what’s traditionally on the exhibit floor.
Most resources that public school advisers suggest for gifted or bright kids are a ‘mile wide and an inch deep’ – they don’t really go into depth on any one area. After traveling to dozens of home school conventions for several years across the country and seeing what math options are out there, I searched for more options than what’s traditionally on the exhibit floor.







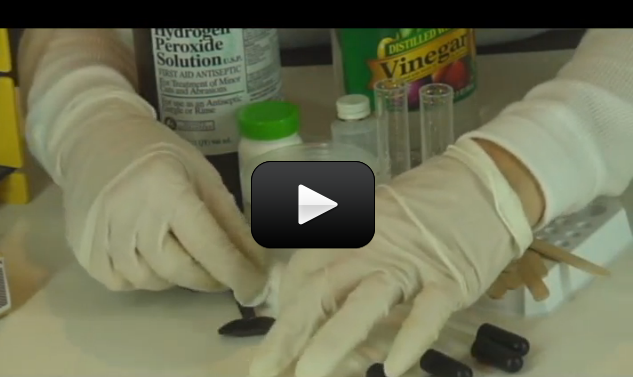
 This experiment below is for advanced students. If you’ve ever wondered why hydrogen peroxide comes in dark bottles, it’s because the liquid reacts with sunlight to decompose from H2O2 (hydrogen peroxide) into H2O (water) and O2 (oxygen). If you uncap the bottle and wait long enough, you’ll eventually get a container of water (although this takes a LOOONG time to get all of the H2O2 transformed.)
This experiment below is for advanced students. If you’ve ever wondered why hydrogen peroxide comes in dark bottles, it’s because the liquid reacts with sunlight to decompose from H2O2 (hydrogen peroxide) into H2O (water) and O2 (oxygen). If you uncap the bottle and wait long enough, you’ll eventually get a container of water (although this takes a LOOONG time to get all of the H2O2 transformed.)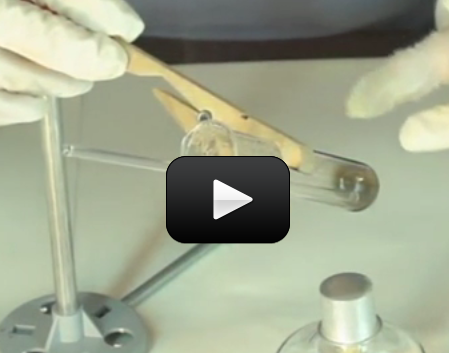

 If you’ve ever owned a fish tank, you know that you need a filter with a pump. Other than cleaning out the fish poop, why else do you need a filter? (Hint: think about a glass of water next to your bed. Does it taste different the next day?)
If you’ve ever owned a fish tank, you know that you need a filter with a pump. Other than cleaning out the fish poop, why else do you need a filter? (Hint: think about a glass of water next to your bed. Does it taste different the next day?)




 I have tried for years to make whole wheat bread from scratch, but my loaves usually wound up as hockey pucks or door stops. Although my house always smelled great, my family could never choke down the crumbs of my latest creation. That’s when I enrolled in a bread-making class. Guess what I found out?
I have tried for years to make whole wheat bread from scratch, but my loaves usually wound up as hockey pucks or door stops. Although my house always smelled great, my family could never choke down the crumbs of my latest creation. That’s when I enrolled in a bread-making class. Guess what I found out?

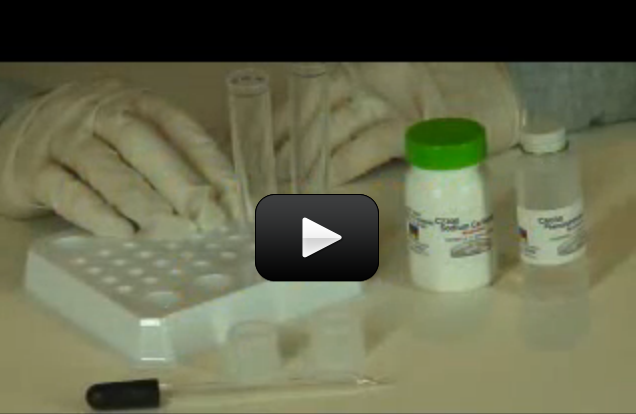
 If you love the idea of mixing up chemicals and dream of having your own mad science lab one day, this one is for you. You are going to mix up each solid with each liquid in a chemical matrix.
If you love the idea of mixing up chemicals and dream of having your own mad science lab one day, this one is for you. You are going to mix up each solid with each liquid in a chemical matrix.

 So this is probably the last chemical in your set you haven’t used… I had to really dig into my ‘bag of tricks’ to find something suitable for you to practice with.
So this is probably the last chemical in your set you haven’t used… I had to really dig into my ‘bag of tricks’ to find something suitable for you to practice with.











 Lyman Spitzer was a theoretical physicist and astronomer who worked on star formation and plasma physics. The scape telescope named after him is equipped with infrared imaging capability that enables the telescope to see through dust and gas clouds to reveal what lies underneath.
Lyman Spitzer was a theoretical physicist and astronomer who worked on star formation and plasma physics. The scape telescope named after him is equipped with infrared imaging capability that enables the telescope to see through dust and gas clouds to reveal what lies underneath.