Have you ever heard of a dollar word search? It’s a special kind of puzzle where the letters in a word add up to a coin value. For example, an A is worth a penny, the letter B is worth two cents, C is worth three cents, and so on. Are you completely confused? That's okay! Just watch the video and I’ll show you how it all works.
This is a neat trick that you can use to really puzzle your friends and family. If someone gives you a three-digit number, you can actually figure out what the end result will be after you've received two additional numbers, but before you actually know what those numbers are. Does this sound confusing? Watch the video and I’ll show you how it works.
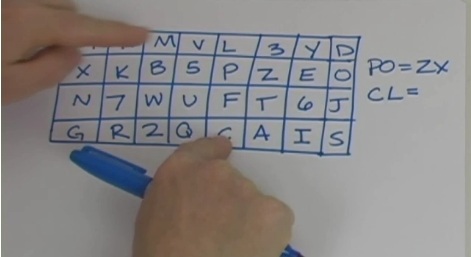
This is a super hard cipher to break. It’s encoded by taking pairs of letters and numbers from a matrix. There are three rules to follow.
- If both letters are in the same row, then use the letters immediately to the right of each other. (Think of the rows as wrapping from the right end back around to that same row’s left end).
- If both letters are in the same column, then use the letters immediately below them. If necessary, the bottom letter wraps back around to the top of the same row.
- If the two letters or numbers are in different rows and in different columns, then each letter is replaced by the letter in the same row that’s also in the same column of the other letter. Basically, you find each intersection of the pair. Use the letter or number below the pair and then the one above the pair.
Play Fair sounds really complicated, but that also makes it a tough code to crack! Watch the video and I’ll explain it all for you.
Please login or register to read the rest of this content.
This is an alternate method of secret writing that’s completely different from encoding and decoding message ciphers. It involves using special inks that are invisible until something is done to make them appear on the paper. There are hundreds of formulas to make these special inks and some formulas even have multiple ways to develop the ink. Some recipes involve special chemicals, but many invisible inks can be made using materials that you have in your home. Watch the video and I’ll share a few recipes and teach you more about this method.
Please login or register to read the rest of this content.
In this video I’ll show you how to use a actual cipher machine called a scytale. This was first used in ancient Greek and Roman times, most notably by the Spartans. To make a scytale, use a cylinder with a piece of paper wrapped around it. Then simply print your message in rows that run along the length of the cylinder. When the paper is unwrapped, the message is scrambled! Watch the video and I’ll show you the trick to proper message decoding.
Please login or register to read the rest of this content.
Numbers really can be huge – some are too big to even imagine! Have you ever seen a million pieces of candy? Or have you ever even tried to count to one million? In this video, we’ll try to figure out about how long it would take just to count to one million. I’ll also show you how to write some really big numbers!
If I said “3!“, would you think the 3 is really excited, or that you have to shout the number?
In fact, it’s a mathematical operation called factorials, and boy are they fun! They may seem complicated at first, but they’re really a very basic concept. The exclamation point behind a number means that you multiply that number by each successively lower number, in order, until you get to one.
So 3! would be 3 x 2 x 1 = 6.
Take a look at the video for an explanation of how factorials work and how they can be used.
Please login or register to read the rest of this content.
 Once in awhile, mathematicians come up against something that really seems impossible on the surface. These seemingly “impossibilities” not only cause them to sit up and take notice, but often to create new rules about the way math works, or at the very least, understand math a little better.
Once in awhile, mathematicians come up against something that really seems impossible on the surface. These seemingly “impossibilities” not only cause them to sit up and take notice, but often to create new rules about the way math works, or at the very least, understand math a little better.
Please login or register to read the rest of this content.
In math, probability is how likely it is that something will occur (or not). Probability is expressed from a range from 0 to 1. A probability of zero means that a thing will definitely not happen – it’s impossible. But a probability of one means that it definitely will happen – it’s certain. Any number larger than 0, but smaller than 1 means that a thing might happen. The number 1/2, or one half, is right in the middle and it means there is a 50/50 chance. Do you think there’s a greater chance for a person to get struck by lightning, or to be hit by a meteorite?
Please login or register to read the rest of this content.
Imagine that you are on a game show with a chance to win a car. There are three doors and the car is behind one of them. You just have to choose the correct door! You can use probability to get an possible advantage in choosing the correct door. Watch the video, and I will explain how it works.
Please login or register to read the rest of this content.
When two blocks of the Earth slip past each other suddenly, that’s what we call an earthquake! From a physics point of view, earthquakes are a release of the elastic potential energy that builds up. Most energy is released as heat, not as shaking, during an earthquake. 90% of all earthquakes happen along the Ring of Fire, which is the active zone that surrounds the Pacific Ocean.
Please login or register to read the rest of this content.
When I was in 10th grade, my teammate and I designed what we thought was pretty clever: a superconductor roller coaster, which we imagined would float effortlessly above its magnetic track. Of course, our roller coaster was only designed on paper, because yttrium barium copper oxide ceramics had only just been discovered by top scientists.
Did you notice how it was smoking in the video? That’s because it was so cold! The usual problem with superconductors is that they need to be incredibly cold in order to exhibit superconductive properties. Yttrium barium copper oxide (YBa2Cu3O7) was the first compound that used liquid nitrogen for cooling, making superconductors a lot less expensive to work with – you no longer needed a cryogenic lab in order to levitate objects above a magnet.
Recently, scientists have found a way to make an amazing superconductor by taking a single crystal sapphire wafer and coating it with a thin (~1µm thick) ceramic material (yttrium barium copper oxide). Normally, the ceramic layer has no interesting magnetic or electrical properties, but that’s when you’re looking at it at room temperature. If you cool this material below -185ºC (-301ºF), it turns out that the ceramic material becomes a superconductor, meaning that it conducts electricity without resistance, with no energy loss. Zero. That’s what makes it a ‘superconductor’.
 To further understand superconductivity, it’s helpful to understand what normally happens to electricity as it flows through a wire. As you may know, energy cannot be created or destroyed, but can be changed from one form to another.
To further understand superconductivity, it’s helpful to understand what normally happens to electricity as it flows through a wire. As you may know, energy cannot be created or destroyed, but can be changed from one form to another.
In the case of wires, some of the electrical energy is changed to heat energy. If you’ve ever touched a wire that had been in use for a while, and discovered it was hot, you’ve experienced this. The heat energy is a waste. It simply means that less electricity gets to its final destination.
This is why superconductivity is so cool (no pun intended.) By cooling things down to temperatures near absolute zero, which is as low as temperatures can get, you can create a phenomenon where electricity flows without having any of it converted to heat.
Why do superconductors float above magnets?
 Scientists also figured out that superconductors and magnetic field really do not like each other. The Meissner effect happens when a superconductor expels all its magnetic fields from inside.
Scientists also figured out that superconductors and magnetic field really do not like each other. The Meissner effect happens when a superconductor expels all its magnetic fields from inside.
However, if you make your superconductor thin enough, you can get the magnetic field to penetrate in discrete quantities (this is real quantum physics now) called flux tubes (the blue lines that go through the disc).
Inside each of the magnetic flux tubes, the superconductivity is destroyed, but the superconductor tries to keep the magnetic tubes pinned in weak areas and any movement of the superconductor itself (like if you pushed it) causes the flux tubes to move, and this is what traps (or locks) the superconductor in midair.
If you’d like to experiment with superconductors yourself, check out this information.
We have done some extensive experiments on taste buds: how they are categorized, what tastes they recognize, and we have even mapped their location on your tongue. But we haven’t yet mentioned this fact: not all people can taste the same flavors!
So today we will check to see if you have a dominant or recessive gene for a distinct genetic characteristic. We’ll do this by testing your reaction to the taste of a chemical called phenylthiocarbamide (or PTC, for short). The interesting thing about PTC is that some people can taste it – and generally have a very adverse reaction. However, some people can’t taste it at all.
Please login or register to read the rest of this content.
Chemical Data & Safe Handling Information Sheet
What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW!
When you’re done storing your chemicals out of reach, watch this video:
Please login or register to read the rest of this content.
Stethoscopes are instruments used to amplify sounds like your heartbeat. Your doctor is trained to use a stethoscope not only to count the beats, but he or she can also hear things like your blood entering and exiting the heart
and its valves opening and closing. Pretty cool!
Today you will make and test a homemade stethoscope. Even though it will be pretty simple, you should still be able to hear your heart beating and your heart pumping. You can also use it to listen to your lungs, just like your doctor does.
Please login or register to read the rest of this content.
Today you will make a calibrated, or marked, container that you will use to measure your lung capacity. You will fill the calibrated container with water, slide a hose into it, take a really deep breath, and blow in the hose. As the air in your lungs enters the container, it will push out the water inside. Just blow as long and as much as you can, then when you flip the bottle over you will be able to read the amount of water you have displaced. If you will subtract the water displaced from the total amount of water in the bottle, the result is your lung capacity.
Please login or register to read the rest of this content.
Chemical Data & Safe Handling Information Sheet
What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW!
When you’re done storing your chemicals out of reach, watch this video:
Please login or register to read the rest of this content.
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH.
Every liquid has a pH. If you pay particular attention to this lab, you will even be able to identify most acids and bases and understand why they do what they do. Acids range from very strong to very weak. The strongest acids will dissolve steel. The weakest acids are in your drink box. The strongest bases behave similarly. They can burn your skin or you can wash your hands with them.
Acid rain is one aspect of low pH that you can see every day if you look for it. This is a strange name, isn’t it? We get rained on all the time. If people were dissolving, if the rain made their skin smoke and burn, you’d think it would make headlines, wouldn’t you? The truth is acid rain is too weak to harm us except in very rare and localized conditions. But it’s hard on limestone buildings.
Please login or register to read the rest of this content.
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum to create the BMW N52 6-cylinder magnesium engine block. Photographers used to use magnesium powder in the camera’s flash before xenon bulbs were available.
Most folks, however, equate magnesium with a burning white flame. Magnesium fires burn too hot to be extinguished using water, so most firefighters use sand or graphite.
We’re going to learn how to (safely) ignite a piece of magnesium in the first experiment, and next how to get energy from it by using it in a battery in the second experiment. Are you ready?
Please login or register to read the rest of this content.
In this lab, we’re going to investigate the wonders of electrochemistry. Electrochemistry became a new branch of chemistry in 1832, founded by Michael Faraday. Michael Faraday is considered the “father of electrochemistry”. The knowledge gained from his work has filtered down to this lab. YOU will be like Michael Faraday. I imagined he would have been overjoyed to do this lab and see the results. You are soooo lucky to be able to take an active part in this experiment. Here’s what you’re going to do…
You will be “creating” metallic copper from a solution of copper sulfate and water, and depositing it on a negative electrode. Copper is one of our more interesting elements. Copper is a metal, and element 29 on your periodic table. It conducts heat and electricity very well.
Many things around you are made of copper. Copper wire is used in electrical wiring. It has been used for centuries in the form of pipes to distribute water and other fluids in homes and in industry. The Statue of Liberty is a wonderful example of how beautiful 180,000 pounds of copper can be. Yes, it is made of copper, and no, it doesn’t look like a penny…..on the surface. The green color is copper oxide, which forms on the surface of copper exposed to air and water. The oxide is formed on the surface and does not attack the bulk of the copper. You could say that copper oxide protects the copper.
Please login or register to read the rest of this content.
If we don’t have salt, we die. It’s that simple. The chemical formula for salt is NaCl. Broken down, we have Na (sodium) and Cl (chlorine). Either one of these can be fatal in sufficient quantities. When chemically combined, these two deadly elements become table salt. What once could kill now keeps us alive. Isn’t chemistry awesome?
Chlorine, element 17, is called a halogen as are all the elements in the 17th row. All halogens have similar chemical properties. They are highly reactive nonmetals, and react easily with most metals. Sodium is a metal, and is bonded with sodium in the table salt used in this lab. Besides being found in salt, chlorine has many uses in our world such as killing bacteria in our water, making plastic, cleaning products, and the list goes on. A very useful chemical, and is among the top ten chemicals produced in the United States. Ever since its discovery in 1774, chlorine has been very useful. It is found in nature in sodium chloride, but in very small concentrations. Seawater, the most abundant source of chlorine, has a concentration of only 19g of chlorine per liter.
Please login or register to read the rest of this content.
Electricity. Chemistry. Nothing in common, have nothing to do with each other. Wrong! Electrochemistry has been a fact since 1774. Once electricity was applied to particular solutions, changes occurred that scientists of the time did not expect.
In this lab, we will discover some of the same things that Farraday found over 300 years ago. We will be there as things tear apart, particles rush about, and the power of attraction is very strong. We’re not talking about dancing, we’re talking about something much more important and interesting….we’re talking about ELECTROCHEMISTRY!
Please login or register to read the rest of this content.
Food and air both enter your body through your mouth, diverging when they reach the esophagus and trachea. Food goes to the gastrointestinal tract through your esophagus and air travels to your lungs via the trachea, or windpipe.
You will be making a model of how your lungs work in this lab. It will include the trachea, lungs, and the diaphragm, which expands and contracts as it fills and empties your lungs.
Please login or register to read the rest of this content.
Ammonia has been used by doctors, farmers, chemists, alchemists, weightlifters, and our families since Roman times. Doctors revive unconscious patients, farmers use it in fertilizer, alchemists tried to use it to make gold, weightlifters sniff it into their lungs to invigorate their respiratory system and clear their heads prior to lifting tremendous loads. At home, ammonia is used to clean up the ketchup you spilled on the floor and never cleaned up.
The ammonia molecule (NH3) is a colorless gas with a strong odor – it’s the smell of freshly cleaned floors and windows. Mom is not cleaning with straight ammonia (it’s gas at room temperature because it boils at -28oF, so the stuff she cleans with is actually ammonium hydroxide, a solution of ammonia and water). Ammonia is found when plants and animals decompose, and it’s also in rainwater, volcanoes, your kidneys (to neutralize excess acid), in the ocean, some fertilizers, in Jupiter’s lower cloud decks, and trace amounts are found in our own atmosphere (it’s lighter than air).
Please login or register to read the rest of this content.
Digestion starts in your mouth as soon as you start to chew. Your saliva is full of enzymes. They are a kind of chemical key that unlock chains of protein, fat, and starch molecules. Enzymes break these chains down into smaller molecules like sugars and amino acids.
In this experiment, we will examine how the enzymes in your mouth help to break down the starch in a cracker. You will test the cracker to confirm starch content, then put it in your mouth and chew it for a long time in order to really let the enzymes do their job. Finally you will test the cracker for starch content and see what has happened as a result of your chewing.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Purple and white colors, making the whitewash that Tom Sawyer used, and produce an exothermic chemical reaction…..does it get any better?
Limewater is one of the compounds we work with in this experiment. Limewater was used in the old days of America. We’re talking about the 80’s…..the 1880’s.
Traveling medicine shows sold what was called “patent medicines”. These usually had no medicinal properties at all. The man in charge, the salesman of the operation, was called a “huckster”. He would have the one of the people gathered around to listen to him blow into limewater. Their exhaled breath contains carbon dioxide, and the lime water turned cloudy, just like in our experiment.
The man would hold up the glass with the cloudy limewater in it and pour in some of his fantastic remedy. As long as the “medicine” was acidic, it would turn the cloudy limewater clear. This was proof that the remedy would cure whatever ailed the person.
Please login or register to read the rest of this content.
This experiment is for advanced students. This is a repeat of the experiment: Can Fish Drown? but now we’re going to do this experiment again with your new chemistry glassware.
The aquarium looked normal in every way, except for the fish. They were breathing very fast and sinking head first to the bottom of the tank. They would sink a few inches, then jerk into proper movement again.
The student had to figure out what was wrong. He had set up the aquarium as an ongoing science project, and it was his responsibility to maintain the fish tank. His grade depended on it.
He went to his mom for help. She looked over the setup. “Have you tested the water?”
A quizzical look on his face, the boy said, “Everything is normal nitrates, nitrites, hardness, alkalinity, and pH. The pH was a little acidic, but not outside the proper range.”
Please login or register to read the rest of this content.
This experiment is for advanced students.
Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done and is being done.
Cars being powered by hydrogen are here, but not on the market yet. Engineers and chemists are always finding new ways to improve the chemical reaction that produces hydrogen and making the vehicles more efficiently use the fuel. Hydrogen fuel is not just easy to make, it is inexpensive, and the “exhaust” is water.
We will generate hydrogen in this lab. We will also see how combustible it is. Just let your imagination wander….just a bit and you will see noiseless cars and trucks zipping along the streets and interstates, carrying people and cargo. The Indianapolis 500 wouldn’t be quite the same, though. “And there they go, roaring, I mean quietly entering turn two…”
Please login or register to read the rest of this content.
This experiment is for advanced students.
In industry, hydrogen peroxide is used in paper making to bleach the pulp before they form it into paper. Biologists, when preparing bones for display, use peroxide to whiten the bones.
At home, 3% peroxide combined with ammonium hydroxide is used to give dark-haired people their desired blonde moment. Peroxide is also used on wounds to clean them and remove dead tissue. Peroxide slows the flow from small blood vessels and oozing in wounds as well.
Please login or register to read the rest of this content.
This experiment is for advanced students.
This time we’re going to use a lot of equipment… really break out all the chemistry stuff. We’ll need all this stuff to generate oxygen with potassium permanganate (KMNO4). We will work with this toxic chemical and we will be careful…won’t we?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Zinc (Zn), is a metal and it is found as element #30 on the periodic table. We need a little zinc to keep our bodies balanced, but too much is very dangerous.
Zinc is just like the common, everyday substance that we all know as di-hydrogen monoxide (which is the chemical name for water). We need water to survive, but too much will kill us.
DHMO: In chemistry, “Di” equals the number 2; hydrogen is H; mono equals the number one; and oxide is derived from oxygen, and its symbol is O. Put these together and you have Di-hydrogen (H2), and mono oxygen (O). Put them together, what do you have? Water!
Please login or register to read the rest of this content.
This experiment is for advanced students.
Lewis and Clark did this same experiment when they reached the Oregon coast in 1805. Men from the expedition traveled fifteen miles south of the fort they had built at the mouth of the Columbia River to where Seaside, Oregon now thrives.
In 1805, however, it was just men from the fort and Indians. They built an oven of rocks. For six weeks, they processed 1,400 gallons of seawater, boiling the water off to gain 28 gallons of salt.
Please login or register to read the rest of this content.
When you exercise your body requires more oxygen in order to burn the fuel that has been stored in your muscles. Since oxygen is moved through your body by red blood cells, exercise increases your heart rate so that the blood can be pumped through your body faster. This delivers the needed oxygen to your muscles faster. The harder you exercise, the more oxygen is needed, so your heart and blood pump even faster still.
Please login or register to read the rest of this content.
This experiment is for advanced students. This lab builds on concepts from the previous carbon dioxide lab.
Limewater….carbon dioxide…indicators. We don’t know too much about these things. Sure, we know a little. Carbon dioxide is exhaled by us and plants need it to grow. Burning fossil fuels produces carbon dioxide.
Indicators…something we observe that confirms to us that something specific is happening. Lime water turns cloudy and forms a precipitate in the presence of carbon dioxide. Blue litmus paper turns red in the presence of an acid. The dog barking at the door and dancing around indicates that you better let the dog out, and quick, to avoid….a pet spill?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Who gets to burn something today? YOU get to burn something today!
You will be working with Zinc (Zn). Other labs in this kit allow us to burn metal, but there is a bit of a twist this time. We will be burning a powder.
Why a powder instead of a solid ribbon or foil as in the other labs? Have you heard of surface area being a factor in a chemical reaction? The more surface area there is to burn, the more dramatic the chemical change. So, with this fact in mind, a powder should burn faster or be more likely to burn than a large solid.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Brimstone is another name for sulfur, and if you’ve ever smelled it burn…..whoa….I’m telling you ….you will see for yourself in this lab. It is quite a smell, for sure. Sulfur is element #6 on the periodic table. Sulfur is used in fertilizer, black powder, matches, and insecticides. In pioneer times sulfur was put into patent medicines and used as a laxative.
To further the evil reputation of sulfur, or brimstone, when sulfur is burned in a coal fired power plant, sulfur dioxide is produced. The sulfur is spewed into the air, where it is reacts with moisture in the air to form sulfuric acid. The clouds get full and need to let go of this sulfuric acid. Down comes the acid rain to wreak havoc on the masonry and plant life below.
Please login or register to read the rest of this content.
This experiment is for advanced students.
ACID!!! The word causes fear to creep in and get our attention.
BASIC!!! The word causes nothing to stir in most of us.
The truth is, a strong acid (pH 0-1) is dangerous, but a strong basic (pH 13-14) is just as dangerous. In this lab, we will get comfortable with the basics of bases and the acidity of acids along with how you can use both and tell the difference between them.
Please login or register to read the rest of this content.
An oxygen and carbon dioxide exchange takes place in your bloodstream. When you breathe air into your lungs it brings in oxygen, which is carried from your lungs by red blood cells in your bloodstream. Cells of your body use the oxygen and carbon dioxide is produced as waste, which is carried by your blood back to your lungs. You exhale and release the C02. You will study this exchange in today’s lab.
You will be using a pH indicator known as bromothymol blue. When you exhale into a baggie, the carbon dioxide will react with water in the bag. This reaction produces carbonic acid, which starts to acidify the water. More breathes in the bag equal more carbon dioxide, which equal a lower (more acidic) pH. You will notice the bromothymol will turn green when the pH of the water is right about 6.8 and it will turn yellow when the pH drops further to 6.0 and lower.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Ever use soap? Sodium hydroxide (NaOH) is the main component in lye soap. NaOH is mixed with some type of fat (vegetable, pig, cow, etc). Scent can be added for the ‘pretty’ factor and pumice or sand can be added for the manly “You’re coming off my hands and I’ll take no guff” factor. Lots of people still make their own soap and they enjoy doing it.
Please login or register to read the rest of this content.
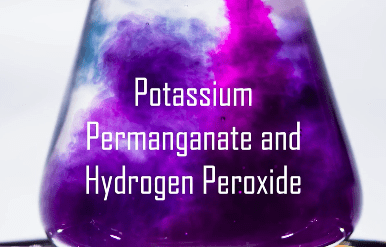
Potassium permanganate (KMnO4) in water turns an intense, deep, purple. It is important in the film industry for aging props and clothing to make them look much older than they are.
Also, artists use it in bone carving. People who carve antlers and bone use KMnO4 to darken the surface of the bone to make it look aged. They make the carving, soak it in potassium permanganate, then carve more, and repeat. The end result is a carving that has a light golden brown color. More dipping will darken the carving even more.
Potassium permanganate is going to undergo a chemical change with this activity. In this experiment, we will be able to witness several indicators of chemical change. Color changes, bubbles from gas generation, temperature change, and color disappearance are all indicators of chemical changes.
Please login or register to read the rest of this content.
How do you know if your brother is stealing your candy? Unwrap a wrapped hard candy that he likes a lot. Roll the candy around in the powdered food dye that matches the candy. (Push the powder into the candy so it “disappears”.) Re-wrap the candy. Set the candy in the place where it usually disappears from. Wait ten minutes after the candy disappears. Find your brother. He will be sporting a new color on his hands and mouth. Dye is hard to remove. It will have to be worn every day at school until it fades away as the skin cells slough off. The dye he now wears is in indicator that he has been taking your candy.
Please login or register to read the rest of this content.
In gas form, element #59 is deadly. However, when iodine is in liquid form, it helps heal cuts and scrapes. The iodine molecule occurs in pairs, not as a single atom (many halogens do this, and it's called a diatomic molecule). It's hard to find iodine in nature, though it's essential for staying healthy... too little iodine in the body takes a heavy toll on how well the brain operates.
A chunk of iodine is blackish-blue, and will sublimate (go from a solid straight to a gas). Iodine is the heaviest element needed by living things. Iodized salt is sodium chloride fortified with iodine to prevent people from not getting enough iodine in their daily diets.
Iodine is found in seaweed (kelp) and seafood as well as vegetables that are grown in dirt that has high iodine levels. People that live inland and do not eat fish often have lower iodine levels than their coastal, fish-eating neighbors. The trick is not to get too much or too little iodine in your diet, because the symptoms of deficiency and excess levels are quite similar.
Starch (like cornstarch) are used as an indicator for detecting iodine in chemistry experiments. When combined with iodine, starch forms a blue-black color in the solution. We're going to do this and many other activities in this lab, because this experiment is actually several labs rolled into one. First, we have to make iodine, store it, and then we get to use it in several experiments. Are you ready?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Zinc and Hydrogen are important elements for all of us. Zinc (Zn) metal is element #30 on the periodic table. Lack of zinc in our diets will delay growth of our bodies and can kill.
Hydrogen gas (H) is element #1 on the periodic table. Hydrogen was discovered in the 1500s. In a pure state, hydrogen combustion (in small quantities) is interesting. In large amounts, mixed with oxygen, the explosion can be devastating.
Please login or register to read the rest of this content.
We've created a video that shows you how to safely do this experiment, although if you're nervous about doing this one, just watch the video and skip the actual experiment.
Bromine is a particularly nasty chemical, so be sure to very carefully follow the steps we've outlined in the video. You MUST do this experiment outdoors. We'll be making a tiny amount to show how the chemical reactions involving bromine work.
Please login or register to read the rest of this content.
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students.
We’ve created a video that shows you how to safely do this experiment, although if you’re nervous about doing this one, just watch the video and skip the actual experiment.
The gas you generate with this experiment is lethal in large doses, so you MUST do this experiment outdoors. We’ll be making a tiny amount to show how the chemical reactions of chlorine and hydrogen work.
Please login or register to read the rest of this content.
How many of these items do you already have? We’ve tried to keep it simple for you by making the majority of the items things most people have within reach (both physically and budget-wise).
Here’s how to use this shopping list: First, look over the list and circle the items you already have on hand. Browse the experiments and note which ones use the materials you already have. Those are the experiments you can start with. After working through the experiments, your child might want to expand and do more activities. Make a note of the materials and put them on your next shopping trip OR order them online using the links provided below.
We’ve tried to keep it simple for you by making the majority of the items things most people have within reach (both physically and budget-wise). Are you ready?
Shopping List for Unit 19: Click here for Shopping List for Unit 19 Online Experiments.
Material List:
Note: These materials listed here are only for the experiments listed in Unit 19 online. (There are different experiments listed in the downloadable Lesson Plan.) If you’d like to do the additional experiments within the Lesson Plan, download the Unit 19 Lesson Plan document and you’ll find the materials list inside.
Robotic Hand
five flexible straws
scrap of cardboard (at least as big as your hand)
five rubber bands
5 feet of string or thin rope
Tasty Tastebuds
1 partner
1 blindfold
1 cup of water
1 plate
1 lemon
2 toothpicks
1 sugar cube
1 salty cracker
1 piece of dark chocolate
1 pencil
Chemical Fingerprinting
1 oz. bottle of baking soda
water
1 sheet of goldenrod paper
1 paper towel
1 magnifying lens
Nerve Tester
1 large paper clip
1 metric ruler
1 partner
Mapping your Tongue
4 cotton swabs
5 wax cups
1 bag of black tea
1 bottle of red vinegar
2 packages of sugar
2 packages of salt
1 microwave
water
1 spoon
1 partner
Detective Boxes
4 shoeboxes with lids
1 soup can
1 pair of scissors
1 sheet of sandpaper
1 sheet of wax paper
1 sheet of flannel fabric
1 plastic bag
1 glue gun
1 pair of gloves
Partners
Finger Thermometers
3 glasses
1 Celsius/Fahrenheit thermometer
1 clock with second hand
hot water
cold water
ice cubes (optional)
room-temperature water
Diffusion
1 onion
1 lemon
1 bottle of ground cinnamon
1 clove of fresh garlic
1 garlic press
1 pile of fresh coffee grounds
1 kitchen knife
1 cutting board
1 variable-speed fan
1 clock with a second hand
Foggy Hands
1 gallon baggie
1 string, 12 inches long
1 assistant
1 clock
Scent Matching
10 small containers with lids
10 cotton balls
1 bottle of lemon juice
1 cup of black coffee
1 bottle of vanilla extract
1 bottle of cinnamon oil
1 bottle of soy sauce
1 black felt marker
1 assistant
Sound Whackers
1 desk
1 metric ruler
Sound Speed
3 baggies, resealable
sand
water
air
1 desktop
1 spoon
1 partner
Big Ears
2 styrofoam cups, 12 oz.
2 styrofoam cups, 32 oz.
1 pair of scissors
1 kitchen timer
Sound Matching
10 film canisters (for 53 mm film rolls)
beans
rice
sawdust (or pencil shavings)
paperclips
pennies
1 black, felt marker
assistant
Water Lens
1 washer, 3/8 inch inside diameter
1 microscope slide
1 container of petroleum jelly
1 piece of newsprint with a lot of type
1 pipette or dropper
water
Camera Eyes
1 dark room
1 light switch
1 partner
1 pencil
Disappearing Frog Experiment
1 frog and dot printout
1 meter stick
1 scrap piece of cardboard
Eyeballoon
1 biconvex lens
1 round balloon, white, 9 inches
1 assistant
1 votive candle
1 black marker
1 book of matches
1 metric ruler
Detecting Temperature Changes
1 measuring cup
1 bottle of calcium chloride
1 bottle of ammonium nitrate
2 resealable baggies
water
Rubber Eggs
4 fresh chicken wing bones, meat removed
1-16 oz. bottle of distilled white vinegar
2-12 oz. plastic cups
1 fresh egg
1 spoon
Cooling and Heating
1 bottle of rubbing alcohol
1 cotton ball
1 liquid crystal thermometer strip
1 cotton glove
Inside Bones
1 toilet paper tube
50-100 straws
1 roll of tape
1 book
Tricking your Muscles
1 partner
1 clock with second hand
Testing Muscle Strength
1 bathroom scale
1 pencil
1 partner
Visual Reflex
1 metric ruler
5 volunteers
Tendon Reflex
1 knee
1 partner
Detecting Plaque
1 4-pack of red disclosing tablets (ask for dentist for a sample pack)
1 clear plastic cup
1 mirror
1 red crayon
water
Testing Spit Samples
1 package of soda crackers
1 5” pie tin
1 craft stick
1 0.5 oz bottle of iodine
1 pre-form tube
plastic pipette or dropper
water
Seeing Your Pulse
1 lump of modeling clay or plastic putty
1 stopwatch
1 coffee stirrer straw
1 partner
Swallowing
1 tennis ball
1 pair of old nylons
1 pair of scissors
Working Lung Model
1 2-liter soda bottle, emptied and cleaned
1 pair of scissors
1 Y valve hose connector
3 round, 9-inch balloons
1 #3 one-hole stopper
1 length of rubber hose, 8-inch
2 rubber bands
1 jar of petroleum jelly
PTC Testing
1 vial of PTC paper
family members
Human Levers
1 body
What’s Your Lung Capacity?
1 2-liter soda bottle
1 black marker, permanent
1 12” length of rubber hose
1 large plastic bowl
1 cup measure
Consuming Oxygen
1 aluminum tart pan
1 votive candle
1 book of matches
1 clear drinking glass, 12 or 16 oz.
1 dime
water
1 pair of goggles
Detecting Carbon Dioxide
1 1 oz. bottle of bromothymol blue
1 straw
1 reasealable baggie
1 bottle of ammonia
1 pipette
water
Heart Rate Monitoring
1 clock with a second hand
1 pencil
Stethoscope
3 12-inch lengths of rubber hose
1 “T” or “Y” connector
1 funnel
Your body moves when muscles pull on the bones through ligaments and tendons. Ligaments attach the bones to other bones, and the tendons attach the bones to the muscles.
If you place your relaxed arm on a table, palm-side up, you can get the fingers to move by pushing on the tendons below your wrist. We’re going to make a real working model of your hand, complete with the tendons that move the fingers! Are you ready?
Please login or register to read the rest of this content.
Did you know that the patterns on the tips of your fingers are unique? It’s true! Just like no two snowflakes are alike, no two people have the same set of fingerprints. In this experiment, you will be using a chemical reaction to generate your own set of blood-red prints.
Please login or register to read the rest of this content.
In this lab, we are going to make an eyeball model using a balloon. This experiment should give you a better idea of how your eyes work. The way your brain actually sees things is still a mystery, but using the balloon we can get a good working model of how light gets to your brain.
Please login or register to read the rest of this content.
The tongue has an ingenious design. Receptors responsible for getting information are separate and compartmentalized. So, different areas on the tongue actually have receptors for different types of tastes. This helps us to separate and enjoy the distinct flavors. In this experiment, you will be locating the receptors for sweet, sour, salty, and bitter on the tongue’s surface.
Please login or register to read the rest of this content.
We now know that odor molecules are diffused throughout a room by the motion of air molecules, which are constantly moving and bumping into them. We also know that warm air moves faster than cold air, and that increasing the movement of the air (like with a fan) will increase the diffusion process.
In this experiment, we look at what happens when the odor molecules find their way into your nose. Your nose has smell cells located in a small area called the olfactory epithelium. We will use them here to match smells with other smells.
Please login or register to read the rest of this content.
In addition to looking pretty neat with all those loops and whirls, your fingertips are great at multitasking. The skin on them has a ton of receptors that help us to gather a lot of information about our environment such as texture, movement, pressure, and temperature.
This experiment will test your ability to determine textures by using touch receptors. You will use shoeboxes with holes cut into them to make texture boxes. Each box will have a textured surface that you can feel, but not see. Through the receptors in your fingers, you will determine whether the surface is rough, waxy, soft, or smooth.
Please login or register to read the rest of this content.
Did you know that your tongue can taste about 10,000 unique flavors? Our tongues take an organized approach to flavor classification by dividing tastes into the four basic categories of sweet, sour, salty, and bitter.
For this experiment, you will need a brave partner! They will be blindfolded and will be attempting to guess foods. Relying only on their sense of taste, they will try to determine what kind of foods you are giving them.
Please login or register to read the rest of this content.
This experiment has two parts. For the first half, you will mix two chemicals that will produce heat and gas. The temperature receptors in your skin will be able to detect the heat. Your ears will detect the gas at it vibrates and escapes its container.
In the second portion you will demonstrate a characteristic in a chemical reaction. For this experiment, it will be an endothermic reaction, which is the absorption of heat energy. This type of reaction is easy to notice because it makes things cold to touch. The chemical you will be using, ammonium nitrate, is actually used in emergency cold packs.
Please login or register to read the rest of this content.
Like sound, light travels in waves. These waves of light enter your eyes through the pupil, which is the small black dot right in the center of your colored iris. Your lens bends and focuses the light that enters your eye. In this experiment, we will study this process of bending light and we will look at the difference between concave and convex lenses.
Please login or register to read the rest of this content.
Skin has another function that it vital to your survival: temperature regulation. Being exposed to high temperatures causes your skin’s pores to open up and release sweat onto your body. This helps cool us off by the resulting process of evaporation.
Your pores will close in extremely cold temperatures. Also, the body stops blood flowing to the skin in order to conserve heat for the important vital organs and their processes.
In this lab, we study the moisture that your skin produces – even when you are not aware of it!
Please login or register to read the rest of this content.
Your fingers have receptors which perform various jobs. In addition to touch, they can detect pressure, texture, and other physical stimuli. One specialized type of receptors is called Ruffini’s receptors. They are good at identifying changes in pressure and temperature. In this experiment, we will test their ability to distinguish between hot and cold temperatures. We are actually going to try and trick your Ruffini endings. Do you think it will work?
Please login or register to read the rest of this content.
Your optic nerve can be thought of as a data cord that is plugged in to each eye and connects them to your brain. The area where the nerve connects to the back of your eye creates a blind spot. There are no receptors in this area at all and if something is in that area, you won’t be able to see it. This experiment locates your blind spot.
Please login or register to read the rest of this content.
Peristalsis is the wavelike movement of muscles that move food through your gastrointestinal tract. The process of digestion begins with chewing and mixing the food with saliva. From there, the epiglottis opens up to deposit a hunk of chewed food (called bolus) into your esophagus – this is the tube that runs from your mouth to your stomach. Since the esophagus is so skinny, the muscles along it must expand and contract in order to move food down. In this activity we will examine that process.
Please login or register to read the rest of this content.
Everything living produces some sort of odor. Flowers use them to entice bees to pollinate them. We know that the tastes of foods are enhanced by the way that they smell. As humans, each of us even has own unique odor.
In this lab, we look at the diffusion of scents. They start in one place, but often end up spread around the room and can be detected by many people.
Please login or register to read the rest of this content.
The eye is a complex structure that detects and focuses light. Light first enters the eye through the cornea, a clear protective layer on the outside of the eye. The pupil, a black opening in the eye, lets light in. In dark rooms, the pupil will become larger, or dilate, in order to let in more light. If the room suddenly becomes bright, the pupil will become smaller. The pupil is surrounded by the brown, blue, grey, or green iris.
After passing through the pupil, light goes to the lens which, like a hand lens, is a clear curved structure that helps focus light on the retina, in the back of the eye. The retina is where the rods and cones are found.
This video is an old instructional film shown to pre-med students in the early 50s you might enjoy watching:
Does it sound impossible to read 25,000 words per minute with a 75% comprehension? Not at all! I learned how to photoread using this cool technique developed by Paul Scheele that I am going to share with you. You’ll be able to digest entire textbooks, articles and newspapers that have been piling up, or read hundreds of emails just a matter of minutes. And no, it’s not ‘speed reading’, and it’s not ‘photographic memory’ either. It’s not magic – but it really works! Anyone who can read a book can learn how to do it.
Most folks read the same way they were taught in grade school – at about 200 words per minute with a 55% comprehension level. And sadly, most remain at this level while stacks of untouched newspapers, magazines, mail, articles, books and reports clutter your living space. When will you ever find the time to read for pleasure with so much to sift through? So many people are trying to cope in the information age using the same reading skills they learned in grade school!
The challenge isn’t whether photoreading is possible, but rather how to incorporate photoreading into your everyday activities, just like eating and sleeping. After I learned how to photoread, I felt much more on top of things in my life, because I had the information I needed to make effective decisions. While I used to spend days reading science textbooks and technical journals, now I only spend minutes per document and I have a clear desk feeling at the end of the day, both at my office and in my home. I can keep up with all the latest daily news in 10 minutes.
The main presumption when picking up a book is that you must read every word in order to understand its message. Not only that, but the book must be read all at one speed: painfully slow.
You know from experience that you don’t have to read every word to get the gist of what a paragraph is talking about. Nor do you have to read an entire textbook at the same speed. We’re going to learn how to super-read, rapid-read, and photoread. Think of it as giving your car a new set of gears: you only had first gear available in your car, and now I’m going to show you how to use 2nd gear, 3rd gear, and overdrive.
Please login or register to read the rest of this content.
Your eyes have two different light receptors located on the back of the eyeball. These are the rods, which see black, white and grays, and the cones, which see color. In order to adapt to the dark, our eyes make a chemical called visual purple. This helps the rods to see and transmit what you see in situations where there is little light.
Your pupils also increase in diameter in the darkness. This allows for a slight increase in the amount of light entering your eye. This combination of visual purple and more light makes it possible for you to see in darker situations.
Please login or register to read the rest of this content.
Levers are classified into three types: first class, second class, or third class. Their class is identified by the location of the load, the force moving the load, and the fulcrum. In this activity, you will learn about the types of levers and then use your body to make each type.
Please login or register to read the rest of this content.
You know that sound comes from vibration which are picked up by the pinna (external part of the ears). Then the vibrations vibrate your tympanic membrane, which in turn vibrates the ossicles and then the cochlea. The cochlea sends information through the auditory nerve and sends it to the brain, which recognizes it as sound.
In this lab, you will testing your ability to sort and match different sounds.
Please login or register to read the rest of this content.
Have you ever held a plastic ruler over the edge of a desk or table and whacked the end of it? If so, you would notice a funny sound. This sound changes if you change the length of the ruler that is hanging over the edge. The sound you hear is made by the ruler’s vibrations.
In this lab, we begin to learn about sound. You know it is collected and deciphered by your ears, but did you also know that all sound is made when something vibrates? It could be a guitar string, vocal chords in your throat, or a plastic ruler that is hanging over the edge of the desk: vibrations make sound.
Please login or register to read the rest of this content.
How do you think animals know we’re around long before they see us? Sure, most have a powerful sense of smell, but they can also hear us first. In this activity, we are going to simulate enhanced tympanic membranes (or ear drums) by attaching styrofoam cups to your ears. This will increase the number of sound waves your ears are able to capture.
Please login or register to read the rest of this content.
It may seem like walking across a balance beam and listening to your favorite song are very different activities, but they both depend on your ears. Ears are the sense organs that control hearing, which is the ability to detect sound. Ears also sense the position of the body and help maintain balance when you walk a balance beam or ride a bike.
Imagine a pebble being dropped into a lake. Waves of water go off in all directions. A similar thing happens when a car driving down the street honks its own. Waves go off from the car in all direction. The difference is that these are not waves of water, but instead are sound waves, which travel through the air. If you are nearby, some of those sound waves make it to your ear.
Here’s a video that shows you how everything works together so you can hear:
The pinna, or outer ear, which is the part of your ear that you can see, gathers up some of the sound waves, sends them down the ear canal, and eventually they strike the eardrum. The eardrum is a thin membrane that vibrates like a drum when the waves hit it. The vibrations pass three tiny bones, called the hammer, anvil, and stirrup, as well as a membrane called the oval window, causing them all to vibrate.
From the oval window, the vibrations go to the cochlea, liquid-filled space lined with hairs. The vibrations make waves in the cochlea’s liquid, just like waves in a pond, causing the hairs to move. The movement of the hairs sends a nerve impulse through the auditory nerve to the brain. The brain interprets the message and “tells” you what you have heard.
This video is an old instructional film shown to pre-med students in the early 50s you might enjoy watching:
Along with hearing, the ears play a major role in balance. Inside the ears are semicircular canals which are lined with hairs and full of liquid. When the body moves in one direction, the liquid in the semicircular canals move, causing the hairs to move. This sends a message to your brain, which gives instructions for the body’s muscles to contract or relax. This keeps you balanced.
There’s a cool video of a camera going inside the ear… watch out for the wax!
Some groups of muscles are stronger than others because each group is designed for a different and specific function. It just makes sense that the muscle groups in our legs would need to be stronger than the ones in our toes.
For this experiment, you will use a bathroom scale to test the strength of various muscle groups.
Please login or register to read the rest of this content.
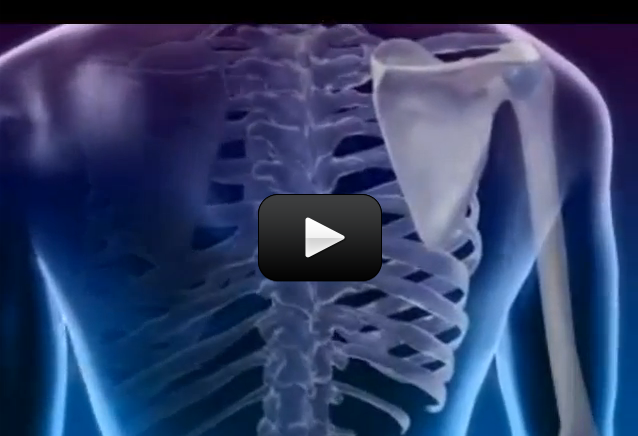
The skeleton is your body’s internal supporting structure. It holds everything together. In addition to providing support, bones act as shock absorbers when you jump, fall, and run. Bones have big responsibilities and so they must be really strong. They also need to be arranged properly for the best support and shock absorption.
In this experiment, we will look at the internal arrangement of the bones holding together your body.
Please login or register to read the rest of this content.
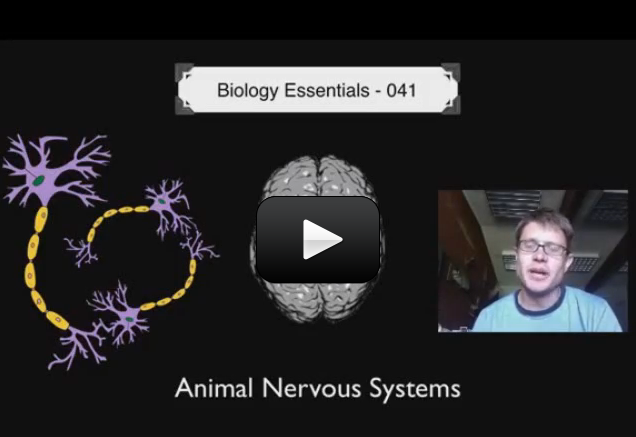
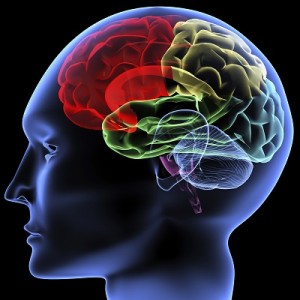 Your body is made of organs, like your heart, lungs or stomach. These organs work together to allow you to breathe, eat, move, and do just about everything else you need to do. Organs working together are called organ systems. Although all organ systems are important, and necessary for us to survive, the most important system might be the nervous system, the system that controls all the others. This system not only controls all the systems of your body but also allows you to learn and use language, senses conditions inside and outside your body and prepares you to fight or flee if you are in a dangerous situation.
Your body is made of organs, like your heart, lungs or stomach. These organs work together to allow you to breathe, eat, move, and do just about everything else you need to do. Organs working together are called organ systems. Although all organ systems are important, and necessary for us to survive, the most important system might be the nervous system, the system that controls all the others. This system not only controls all the systems of your body but also allows you to learn and use language, senses conditions inside and outside your body and prepares you to fight or flee if you are in a dangerous situation.
This is a video about the more advanced concepts about neurons, nerves, and signals:
Imagine you walk into your home one night and it’s pitch black. You quickly flick on a light switch and the room is immediately lit up. You may have never thought about it, but it’s kind of amazing that this happens. Even though it may be a long distance from the light switch to the actual light, the light comes on.
Fortunately, messages in your nervous system also travel at the speed of electricity. The nervous system is made of bundles of nerve cells called nerves. Nerve cells called neurons send messages, called nerve impulses, throughout the body. Since these messages are electrical impulses, they travel very quickly. Neurons are also covered with a fatty covering called myelin which insulates the neuron, like the plastic on the outside of a wire, allowing the nerve impulse to travel even faster.
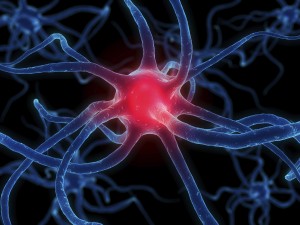 Look at the shape of the neuron. These cells are the perfect shape for their job of sending messages all over the body. The small extensions on the end of the cell are called dendrites and get nerve impulses from other cells. The longer extension at the other end of the cell is called an axon and sends nerve impulses to other cells. The central part of the cell, between the dendrites and axon, is called the cell body. This part of the cell contains the nucleus and all the organelles, which are small structures inside the cell. The place where the axon of a nerve cell meets the dendrites of another nerve cell or another type of cell is called a synapse.
Look at the shape of the neuron. These cells are the perfect shape for their job of sending messages all over the body. The small extensions on the end of the cell are called dendrites and get nerve impulses from other cells. The longer extension at the other end of the cell is called an axon and sends nerve impulses to other cells. The central part of the cell, between the dendrites and axon, is called the cell body. This part of the cell contains the nucleus and all the organelles, which are small structures inside the cell. The place where the axon of a nerve cell meets the dendrites of another nerve cell or another type of cell is called a synapse.
Neurons can be classified, or put into groups, based on their job in the body. Sensory neurons carry messages from the organs to the brain and spinal cord. Motor neurons carry messages from the brain and spinal cord to organs, glands, and muscles.
Have you ever wondered how cells communicate? Here’s a video for advanced, curious students:
As you can see, the nervous system does many things and has many parts. Consider the chart below, which lists the divisions of the system. Each part of the system plays a unique and important role in allowing the other body systems to function.
Our sense of touch provides us with information that helps us to process and explore our world. Nerves play an important part in the sense of touch by being the wires that carry signals from the skin to the brain. But the body has a plan in place so that our brains don’t get overwhelmed with too much information. This plan is a lot like a blueprint for wiring a house. Just like a house has light switches and electrical outlets in strategic locations, our bodies have touch receptors of various numbers based on their location. In this lab, we will explore an arm to determine where the highest concentrations of nerves are in that limb.
Please login or register to read the rest of this content.
Bones are made up of several parts; bone marrow (red and yellow), spongy bone, compact bone, and the periosteum. Bone marrow makes blood cells. Red blood cells are made in the red marrow, while white blood cells are made in the yellow marrow. When babies are born they only have red marrow. Spongy bone is a light, spongy type of bone found inside bones. Compact bone, on the other hand, is hard and makes up the outer layer of bones. The compact bone layer is covered by a thin white membrane called the periosteum.
Bones begin growing very early, and stop growing between the ages of 18-25. At about eight weeks of development, we form a skeleton of cartilage and other connective tissues. As we grow up, the cartilage becomes bone. Normally, we have all of our bones by our early twenties. We still keep some of the cartilage in areas like our nose and ears.
Joints are essential in how we move. Bones work as levers and the joints work as the fulcrums—making our movement easier. Some joints are fixed; for example, many in the skull. Some allow only little movement; for example, the vertebrae which make up the backbone. Lastly, there are movable joints; for example, our knees and elbows. These make up the three classes of joints: fixed joints, partly movable joints, and movable joints.
The keys to keeping the skeletal system healthy are: eating well, getting exercise, and taking care of injuries to the skeletal system.
Eating a good balanced diet is very important for overall health, but making sure to get specific nutrients help ensure a healthy skeletal system throughout your life! Those nutrients are: calcium and vitamin D. 1300mg of Calcium is recommended (one cup of milk has about 300mg of Calcium) and 200IU of vitamin D (31/2 ounces of cooked salmon is about 360IU of vitamin D). Calcium can be found in dairy products as well as broccoli and cabbage. Your skin makes vitamin D when exposed to sunlight. Additionally, fish is rich in vitamin D.
It’s important to get out an exercise to maintain a healthy skeletal system. When we exercise we put stress on our bones and stimulate them to stay strong. Exercising also keeps the muscles which work with the bones strong. Just remember to stretch and wear all the appropriate safety gear.
Lastly, if an injury occurs—a bone breaks or a ligament tears, for example—it’s important to see a medical professional as soon as possible. Otherwise, the skeletal system may not heal properly!
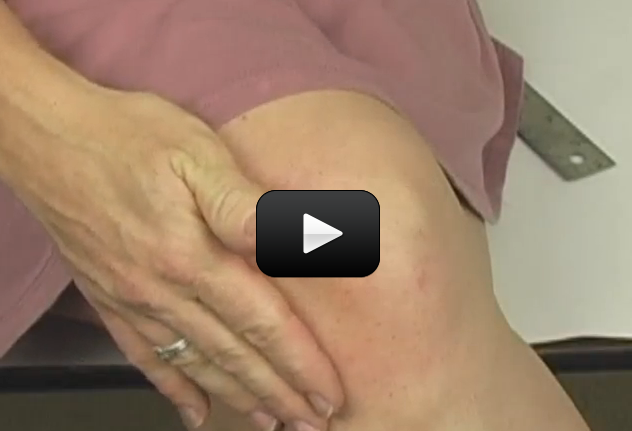
Involuntary responses are ones that you can’t control, but they are usually in place to help with survival. One good example is when you touch something hot. Your hand does not take the time to send a message to your brain and then have the brain tell your hand to pull away. By then, your hand might be seriously hurt! Instead, your body immediately removes your hand in order to protect it from further harm.
Today you will test an involuntary reflex by using the tendon reflex test. A thick, rubbery band called the patellar tendon holds your knee cap in place. Having one leg on top of the other not only stretches the tendon, but it also makes it possible to see a reaction. You can test the reflex by giving your tendon a tap and watching what happens.
Please login or register to read the rest of this content.
Our cells are happiest when they are in their normal or “home” state. This is a state in which the temperature, the concentrations of molecules, and molecules being produced are all at the levels at which they normally function. This “normal” or “home” state is called homeostasis.
Our cells—and the tissues and organs they constitute—work hard to maintain homeostasis. We can see this in action. When it is cold out and we shiver, that’s our body trying to get the temperature up to normal levels. When it’s hot out and we sweat, that’s our body trying to get the temperature down to normal levels. We see our body trying to maintain homeostasis when we feel hungry, or thirsty. Homeostasis is an important characteristic of living things. If you were in the desert, your body would be working hard to maintain homeostasis—despite the high temperature and the lack of water.
There are many different types of cells in the body, but all of them work to maintain homeostasis. For example, there are specific muscle cells for muscles, specific heart cells for the heart, specific pancreas cells for the pancreas, and skin cell cells making up the skin. The different cell types differ in how they function. They all work together to make sure the body functions normally.
A tissue is composed of specific cells performing the same function. An organ is made up of two or more types of tissues working together. Organs which work together form organ systems. Organ systems work together to maintain homeostasis.
So, how many types of tissues are there? There are four main types of tissue. Tissues are groups of cells which together form specific functions. These types are: epithelial tissue, nervous tissue, muscle tissue, and connective tissue. Epithelial tissue is found in tightly packed surface layers; such as the skin, as well as the lining of the digestive system and the lining of the mouth and nose. Nervous tissue is responsible for relaying information. All together the nervous tissues form the nervous system. The nervous system includes the sensory nerves in the body, nerves in the spinal cord, and the brain.
There are three types of muscle tissue; smooth muscle, skeletal muscle, and cardiac muscle. All three cell types have filaments which change the size. Bone, cartilage, and tendon tissues are examples of connective tissue. Connective tissue connects one part of the body to another and is involved in structural support.
The low blood temperature sends a message to the brain which then sends a message to the adrenal gland, which increases the blood temperature by raising the metabolism. The raised blood temperature then signals to the brain that it’s time to stop sending the message to the adrenal gland. A key way organ systems maintain homeostasis is via a negative feedback loop.
A negative feedback loop simply means that the result is a signal to stop. For example, if you haven’t eaten for a while your body will sense that your blood sugar is low. The low blood sugar acts as a signal for the body to start releasing sugar into your blood. However, once the blood sugar levels are back to normal—homeostasis has been reestablished—that normalcy acts as a signal for the body to stop releasing sugar into your blood.
Many problems can occur if these negative feedback loops do not function properly. For example, diabetes is a disease which results from the blood sugar negative feedback loop functioning abnormally.
The buildup of things like food and bacteria where your gums and teeth meet, and also between your teeth, is called plaque. Where plaque lives is also where the bacteria turns the sugar in your mouth into harmful acids that attack your teeth’s enamel and can lead to gum disease. Regular brushing is a great way to remove plaque and keep your mouth healthy.
Please login or register to read the rest of this content.
Earth, our home in space, has supported life for a long time. But with a growing human population, people are having a greater effect on the environment than ever before. Together we must learn about the problems facing our environment and work to protect the earth.
There are many ways we can work together to protect the earth. We can ask adults to use more fuel-efficient cars (cars that get more miles per gallon of gasoline). We can ride bikes or walk instead of getting rides in cars. We can recycle aluminum, paper, plastic, and glass, and we can plant trees. We can save energy by turning off lights when they are not in use. We can save energy by not keeping rooms and buildings too hot in the winter or too cold in the summer. Another way we can help the earth is to learn more about the environment.
Please login or register to read the rest of this content.
Let’s see how much you’ve picked up with these experiments and the reading – answer as best as you can. (No peeking at the answers until you’re done!) Just relax and see what jumps to mind when you read the question. You can also print these out and jot down your answers in your science notebook.
Please login or register to read the rest of this content.
Let’s see how you did! If you didn’t get a few of these, don’t let it stress you out – it just means you need to play with more experiments in this area. We’re all works in progress, and we have our entire lifetime to puzzle together the mysteries of the universe!
Here’s printer-friendly versions of the exercises and answers for you to print out: Simply click here for printable questions and answers.
Answers:
Please login or register to read the rest of this content.
How do astronomers find planets around distant stars? If you look at a star through binoculars or a telescope, you’ll quickly notice how bright the star is, and how difficult it is to see anything other than the star, especially a small planet that doesn’t generate any light of its own!
Please login or register to read the rest of this content.
Without the sun, there would be no life on Earth. The sun warms the earth, generates wind, and carries water into the air to produce rain and snow. The energy of the sun provides sunlight for all the plant life on our planet, and through plants provides energy for all animals.
The sun is like a giant furnace in which hydrogen nuclei (atoms without electrons) are constantly smashed together to form helium nuclei. This process is called nuclear fusion. In this process, 3.6 billion kilograms (8 billion pounds) of matter are converted to pure energy every second. The temperature in the sun exceeds 15 million degrees.
Nuclear fusion is one kind of energy. Other forms of energy include: mechanical energy, heat, electrical energy, chemical energy, and light. Mechanical energy is the energy of organized motion, such as a turning wheel. Heat is the energy of random motion, such as a cup of hot water. Electrical energy is the energy of moving charged particles or electrons, such as a current in a wire. Chemical energy is the energy stored in bonds that hold atoms together. Light is any form of electromagnetic waves, such as X rays, microwaves, radio waves, ultraviolet light, or visible light.
Energy can be converted from one from to another. For example, the nuclear energy of the sun is converted to light, which goes through space to the earth. Solar collectors of mirrors can be used to focus some of that light to heat water to steam. This steam can be used to turn a turbine, which can power a generator to produce electricity.
Most of our energy needs are met by burning fossil fuels such as coal, oil, gasoline, and natural gas. The chemical energy stored in these substances is released by burning these fuels. When fossil fuels burn, they combine with oxygen in the air and produce heat and light.
Fossil fuels are not renewable. When they are used up, they are gone forever. However, renewable energy sources such as wind, sun, geothermal, biomass and water power are renewable. They can be used over and over to generate the energy to run our society.
Tremendous amounts of renewable energy are available. For example, the solar energy that falls on just the road surfaces in the United States is equal to the entire energy needs of the country. Although there are sufficient amounts of renewable energy, we must improve our methods of collecting, concentrating, and converting renewable energy into useful forms.
In the following experiments, you will learn something about the amount of energy the sun produces at the earth’s surface and how heat energy can be stored.
Please login or register to read the rest of this content.
Temperature is a measure of the average hotness of an object. The hotter an object, the higher its temperature. As the temperature is raised, the atoms and molecules in an object move faster. The molecules in hot water move faster than the molecules in cold water. Remember that the heat energy stored in an object depends on both the temperature and the amount of the substance. A smaller amount of water will have less heat energy than a larger amount of water at the same temperature.
Increasing the temperature of a large body of water is one way to store heat energy for later use. A large container filled with salt water, called brine, may be used to absorb heat energy during the day when it is warm. This energy will be held in the salt water until the night when it is cooler. This stored heat energy can be released at night to warm a house or building. This is one way to store the sun’s heat energy until it is needed.
Solar ponds are used to store energy from the sun. Temperatures close to 100°C (212°F) have been achieved in solar ponds. Solar ponds contain a layer of fresh water above a layer of salt water. Because the salt water is heavier, it remains at the bottom of the pond-even as it gets quite hot. A black plastic bottom helps absorb solar energy from sunlight. The water on top serves to insulate and trap the heat in the pond.
In a fresh water pond, as the water on the bottom is heated from sunlight, the hot water becomes lighter and rises to the top of the pond. This convection or movement of hot water to the top tends to carry away excess heat. However, in a salt water pond, there is no convection so heat is trapped. In Israel a series of salt water, solar ponds were developed around the Dead Sea. The heat stored in these solar ponds has been used to run turbines and generate electricity.
Please login or register to read the rest of this content.
The United States has large reserves of coal, natural gas, and crude oil which is used to make gasoline. However, the United States uses the energy of millions of barrels of crude oil every day, and it must import about half its crude oil from other countries.
Burning fossil fuels (oil, coal, gasoline, and natural gas) produces carbon dioxide gas. Carbon dioxide is one of the main greenhouse gases that may contribute to global warming. In addition, burning coal and gasoline can produce pollution molecules that contribute to smog and acid rain.
Using renewable energy-such as solar, wind, water, biomass, and geothermal-could help reduce pollution, prevent global warming, and decrease acid rain. Nuclear energy also has these advantages, but it requires storing radioactive wastes generated by nuclear power plants. Currently, renewable energy produces only a small part of the energy needs of the
United States. However, as technology improves, renewable energy should become less expensive and more common.
Hydropower (water power) is the least expensive way to produce I electricity. The sun causes water to evaporate. The evaporated water falls to the earth as rain or snow and fills lakes. Hydropower uses water stored in lakes behind dams. As water flows through a dam, the falling water turns turbines that run generators to produce electricity.
Currently, geothermal energy (heat inside the earth), biomass (energy from plants), solar energy (light from concentrated sunlight), and wind are being used to generate electricity. For example, in California there are more than sixteen thousand (16,000) wind turbines that generate enough power to supply a city the size of San Francisco with electricity.
In addition to producing more energy, we can also help meet our energy needs through conservation. Conservation means using less energy and using it more efficiently.
In the following experiments, you will use wind to do work, examine how batteries can store energy, and see how insulation can save energy.
Please login or register to read the rest of this content.

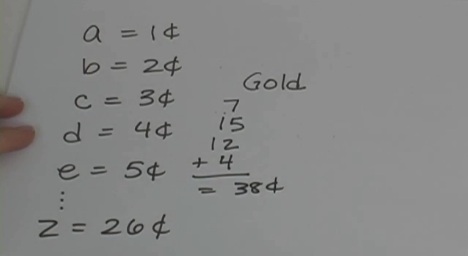






















































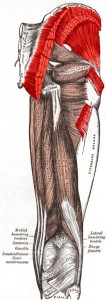
 The muscles in your body allow you to move. In this lab we will do two quick experiments to explore how your muscles work.
The muscles in your body allow you to move. In this lab we will do two quick experiments to explore how your muscles work.