When people mention the word “hydraulics”, they could be talking about pumps, turbines, hydropower, erosion, or river channel flow. The term “hydraulics” means using
fluid power, and deals with machinesand devices that use liquids to move, lift, drive, and shove things around.
Liquids behave in certain ways: they are incompressible, meaning that you can’t pack the liquid into a tighter space than it already is occupying.
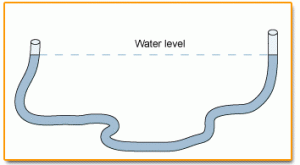
If you've ever filled a tube partway with water and moved it around, you've probably noticed that the water level will remain the same on either side of the tube.
However, if you add pressure to one end of the tube (by blowing into the tube), the water level will rise on the opposite side. If you decrease the pressure (by blowing
across the top of one side), the water level will drop on the other side.
In physics, this is defined through Pascal's law, which tells us how the pressure applied to one surface can be transmitted to the other surface. As liquids can't be squished, whatever happens on one surface affects what occurs on the other. Examples of this effect include siphons, water towers, and dams. Scuba divers know that as they dive 30 feet underwater, the pressure doubles. This effect is also show in hydraulics - and more importantly, in the project we're about to do!
But first, let's understand what's happening with liquids and pressure:
Here’s an example: If you fill a glass full to the brim with water, you reach a point where for every drop you add on top, one drop will fall out. You simply can’t squish any more water molecules into the glass without losing at least the same amount. Excavators, jacks, and the brake lines in your car use hydraulics to lift huge amounts of weight, and the liquid used to transfer the force is usually oil at 10,000 psi.
Air, however,
is compressible. When car tires are inflated, the hose shoves more and more air inside the tire, increasing the pressure (amount of air molecules in the tire). The more air you stuff into the tire, the higher the pressure rises. When machines use air to lift, move, spin, or drill, it’s called “pneumatics”. Air tools use compressed air or pure gases for pneumatic power, usually pressurized to 80-100 psi.
Different systems require either hydraulics or pneumatics. The advantage to using hydraulics lies in the fact that liquids are not compressible. Hydraulic systems minimize the “springy-ness” in a system because the liquid doesn’t absorb the energy being transferred, and the working fluids can handle much heavier loads than compressible gases. However, oil is flammable, very messy, and requires electricity to power the machines, making pneumatics the best choice for smaller applications, including air tools (to absorb excessive forces without injuring the user).
We're going to build our own hydraulic-pneumatic machine. Here's what you need to do:
Please
login or
register to read the rest of this content.


































 Find a smooth, cylindrical support column, such as those used to support open-air roofs for breezeways and outdoor hallways (check your local public school or local church). Wind a length of rope one time around the column, and pull on one end while three friends pull on the other in a tug-of-war fashion.
Experiment with the number of friends and the number of winds around the column. Can you hold your end with just two fingers against an entire team of football players? You bet!
Please
Find a smooth, cylindrical support column, such as those used to support open-air roofs for breezeways and outdoor hallways (check your local public school or local church). Wind a length of rope one time around the column, and pull on one end while three friends pull on the other in a tug-of-war fashion.
Experiment with the number of friends and the number of winds around the column. Can you hold your end with just two fingers against an entire team of football players? You bet!
Please 
 Let's take a good look at Newton's Laws in motion while making something that flies off in both directions. This experiment will pop a cork out of a bottle and make the cork fly go 20 to 30 feet, while the vehicle moves in the other direction!
This is an outdoor experiment. Be careful with this, as the cork comes out with a good amount of force. (Don’t point it at anyone or anything, even yourself!)
Here's what you need to find:
Please
Let's take a good look at Newton's Laws in motion while making something that flies off in both directions. This experiment will pop a cork out of a bottle and make the cork fly go 20 to 30 feet, while the vehicle moves in the other direction!
This is an outdoor experiment. Be careful with this, as the cork comes out with a good amount of force. (Don’t point it at anyone or anything, even yourself!)
Here's what you need to find:
Please 
 Every wonder why you have to wear a seat belt or wear a helmet? Let's find out (safely) by experimenting with a ball.
You will need to find:
Every wonder why you have to wear a seat belt or wear a helmet? Let's find out (safely) by experimenting with a ball.
You will need to find:

 Hovercraft transport people and their stuff across ice, grass, swamp, water, and land. Also known as the Air Cushioned Vehicle (ACV), these machines use air to greatly reduce the sliding friction between the bottom of the vehicle (the skirt) and the ground. This is a great example of how lubrication works – most people think of oil as the only way to reduce sliding friction, but gases work well if done right.
Hovercraft transport people and their stuff across ice, grass, swamp, water, and land. Also known as the Air Cushioned Vehicle (ACV), these machines use air to greatly reduce the sliding friction between the bottom of the vehicle (the skirt) and the ground. This is a great example of how lubrication works – most people think of oil as the only way to reduce sliding friction, but gases work well if done right.
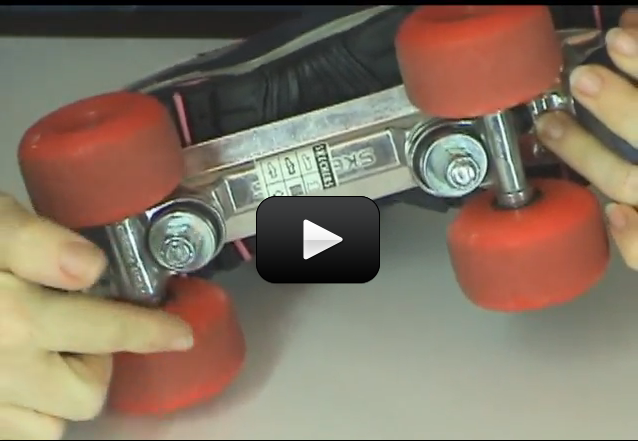



 This experiment is for advanced students.It’s time for the last lesson of mechanics. After all this time, you now have a good working knowledge of the rules that govern almost all movement on this planet and beyond!! This lesson we get to learn about things crashing into one another!! Isn’t physics fun?! We are going to learn about impulse and momentum.
This experiment is for advanced students.It’s time for the last lesson of mechanics. After all this time, you now have a good working knowledge of the rules that govern almost all movement on this planet and beyond!! This lesson we get to learn about things crashing into one another!! Isn’t physics fun?! We are going to learn about impulse and momentum.

 This is a quick and easy demonstration of how to teach Newton’s laws with minimal fuss and materials. All you need is a wagon, a rock, and some friends. We’re going to do a few totally different experiments using the same materials, though, so keep up with the changes as you read through the experiment.
This is a quick and easy demonstration of how to teach Newton’s laws with minimal fuss and materials. All you need is a wagon, a rock, and some friends. We’re going to do a few totally different experiments using the same materials, though, so keep up with the changes as you read through the experiment.








 This experiment is for Advanced Students. For ages, people have been hurling rocks, sticks, and other objects through the air. The trebuchet came around during the Middle Ages as a way to break through the massive defenses of castles and cities. It’s basically a gigantic sling that uses a lever arm to quickly speed up the rocks before letting go. A trebuchet is typically more accurate than a catapult, and won’t knock your kid’s teeth out while they try to load it.
This experiment is for Advanced Students. For ages, people have been hurling rocks, sticks, and other objects through the air. The trebuchet came around during the Middle Ages as a way to break through the massive defenses of castles and cities. It’s basically a gigantic sling that uses a lever arm to quickly speed up the rocks before letting go. A trebuchet is typically more accurate than a catapult, and won’t knock your kid’s teeth out while they try to load it.