This experiment is for advanced students.
Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done and is being done.
Cars being powered by hydrogen are here, but not on the market yet. Engineers and chemists are always finding new ways to improve the chemical reaction that produces hydrogen and making the vehicles more efficiently use the fuel. Hydrogen fuel is not just easy to make, it is inexpensive, and the “exhaust” is water.
We will generate hydrogen in this lab. We will also see how combustible it is. Just let your imagination wander….just a bit and you will see noiseless cars and trucks zipping along the streets and interstates, carrying people and cargo. The Indianapolis 500 wouldn’t be quite the same, though. “And there they go, roaring, I mean quietly entering turn two…”
Please login or register to read the rest of this content.

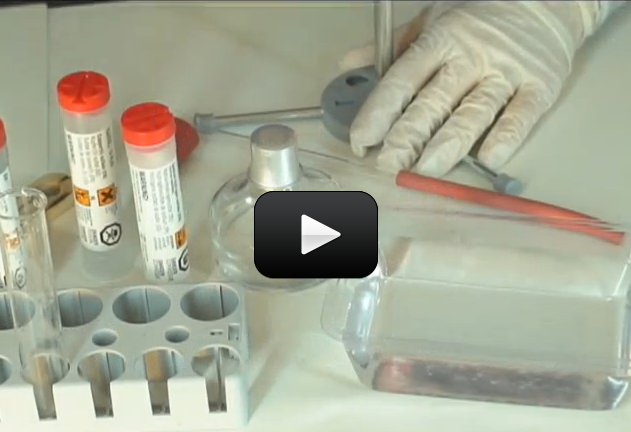

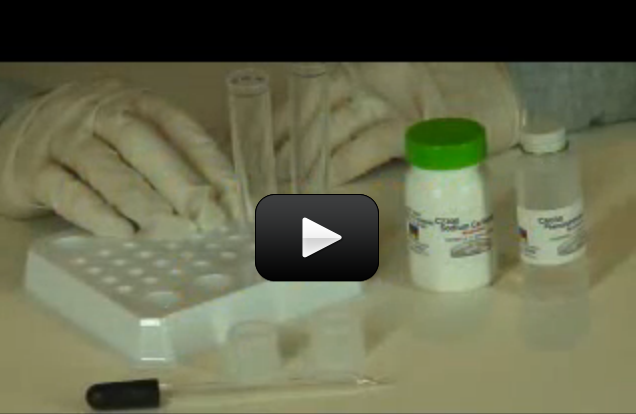

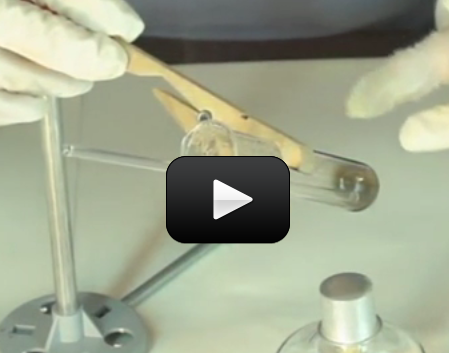





















 Can we really make crystals out of soap? You bet! These crystals grow really fast, provided your solution is properly saturated. In only 12 hours, you should have sizable crystals sprouting up.
Can we really make crystals out of soap? You bet! These crystals grow really fast, provided your solution is properly saturated. In only 12 hours, you should have sizable crystals sprouting up.
 The atoms in a solid, as we mentioned before, are usually held close to one another and tightly together. Imagine a bunch of folks all stuck to one another with glue. Each person can wiggle and jiggle but they can’t really move anywhere.
The atoms in a solid, as we mentioned before, are usually held close to one another and tightly together. Imagine a bunch of folks all stuck to one another with glue. Each person can wiggle and jiggle but they can’t really move anywhere.


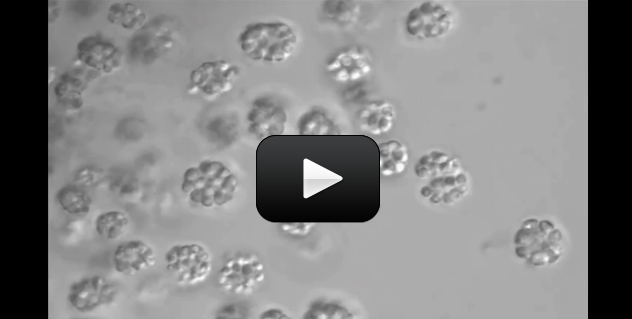
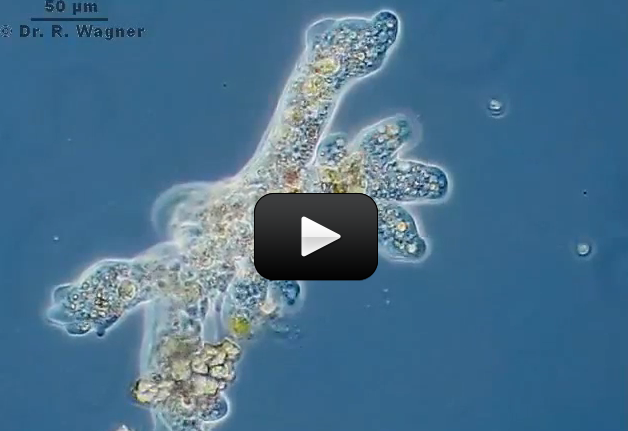
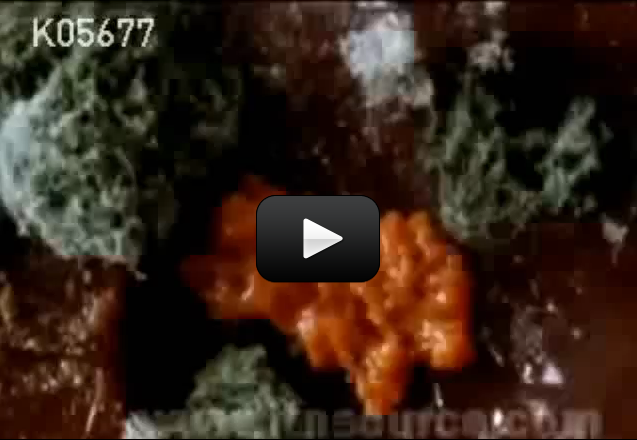
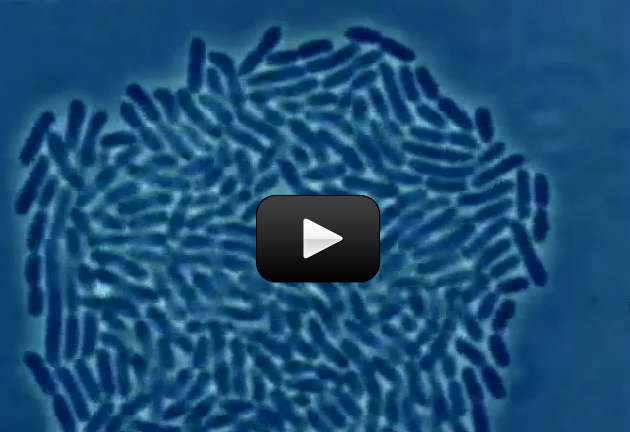
 Some organisms, like bacteria, consist of only one cell. Other organisms, like humans, consist of trillions of specialized cells working together. Even if organisms look very different from each other, if you look close enough you’ll see that their cells have much in common.
Some organisms, like bacteria, consist of only one cell. Other organisms, like humans, consist of trillions of specialized cells working together. Even if organisms look very different from each other, if you look close enough you’ll see that their cells have much in common.







 If you have a backyard garden, be sure to give it plenty of sunshine, water, and garbage.
If you have a backyard garden, be sure to give it plenty of sunshine, water, and garbage.



 If you’ve ever eaten fruits or vegetables (and let’s hope you have), you have benefited from plants as food. Of course, the plants we eat have been highly modified by growers to produce larger and sweeter fruit, or heartier vegetables.
If you’ve ever eaten fruits or vegetables (and let’s hope you have), you have benefited from plants as food. Of course, the plants we eat have been highly modified by growers to produce larger and sweeter fruit, or heartier vegetables.
 Alexander Graham Bell developed the telegraph, microphone, and telephone back in the late 1800s. We’ll be talking about electromagnetism in a later unit, but we’re going to cover a few basics here so you can understand how loudspeakers transform an electrical signal into sound.
Alexander Graham Bell developed the telegraph, microphone, and telephone back in the late 1800s. We’ll be talking about electromagnetism in a later unit, but we’re going to cover a few basics here so you can understand how loudspeakers transform an electrical signal into sound.


 Imagine you have two magnets. Glue one magnet on an imaginary record player (or a ‘lazy susan’ turntable) and hold the other magnet in your hand. What happens when you bring your hand close to the turntable magnet and bring the north sides together?
Imagine you have two magnets. Glue one magnet on an imaginary record player (or a ‘lazy susan’ turntable) and hold the other magnet in your hand. What happens when you bring your hand close to the turntable magnet and bring the north sides together?
 This experiment is for advanced students. If you’ve attempted the
This experiment is for advanced students. If you’ve attempted the 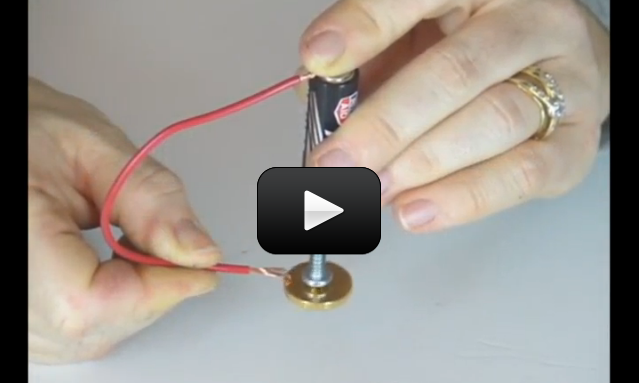





 There are two ways to create a magnetic field. First, you can wrap wire around a nail and attach the ends of the wire to a battery to make an electromagnet. When you connect the battery to the wires, current begins to flow, creating a magnetic field. However, the magnets that stick to your fridge are neither moving nor plugged into the electrical outlet – which leads to the second way to make a magnetic field: by rubbing a nail with a magnet to line up the electron spin. You can essential “choreograph” the way an electron spins around the atom to increase the magnetic field of the material. This project is for advanced students.
There are two ways to create a magnetic field. First, you can wrap wire around a nail and attach the ends of the wire to a battery to make an electromagnet. When you connect the battery to the wires, current begins to flow, creating a magnetic field. However, the magnets that stick to your fridge are neither moving nor plugged into the electrical outlet – which leads to the second way to make a magnetic field: by rubbing a nail with a magnet to line up the electron spin. You can essential “choreograph” the way an electron spins around the atom to increase the magnetic field of the material. This project is for advanced students.



 Wouldn’t it be cool to have an alarm sound each time someone opened your door, lunch box, or secret drawer? It’s easy when you use a reed switch in your circuit! All you need to do it substitute this sensor for the
Wouldn’t it be cool to have an alarm sound each time someone opened your door, lunch box, or secret drawer? It’s easy when you use a reed switch in your circuit! All you need to do it substitute this sensor for the 




 The smallest thing around is the atom, which has three main parts – the core (nucleus) houses the protons and neutrons, and the electron zips around in a cloud around the nucleus.
The smallest thing around is the atom, which has three main parts – the core (nucleus) houses the protons and neutrons, and the electron zips around in a cloud around the nucleus.
 It’s easy to use chemistry to generate electricity, once you understand the basics. With this experiment, you’ll use aluminum foil, salt, air, and a chemical from an aquarium to create an air battery. This experiment is for advanced students.
It’s easy to use chemistry to generate electricity, once you understand the basics. With this experiment, you’ll use aluminum foil, salt, air, and a chemical from an aquarium to create an air battery. This experiment is for advanced students.





 Electrical circuits are used for all kinds of applications, from blenders to hair dryers to cars. And games! Here’s a quick and easy game using the principles of conductivity.
Electrical circuits are used for all kinds of applications, from blenders to hair dryers to cars. And games! Here’s a quick and easy game using the principles of conductivity.

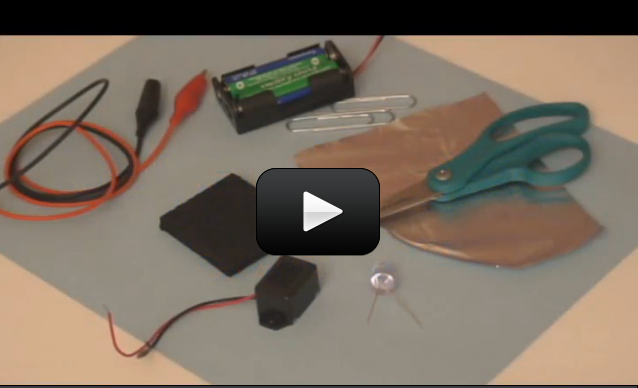

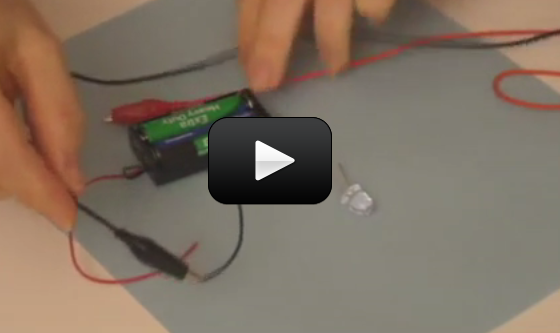
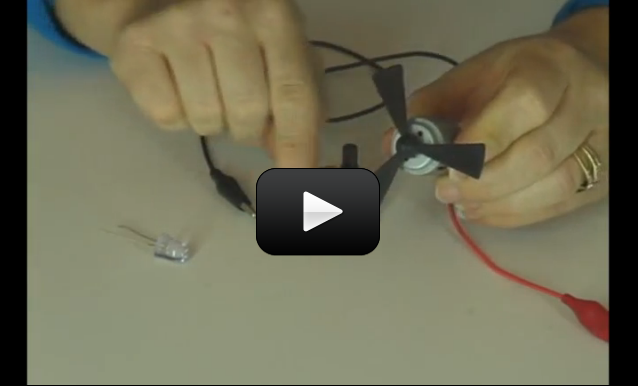
 So now you know how to hook up a
So now you know how to hook up a 
 One of the most useful tools a scientist can have! A
One of the most useful tools a scientist can have! A 
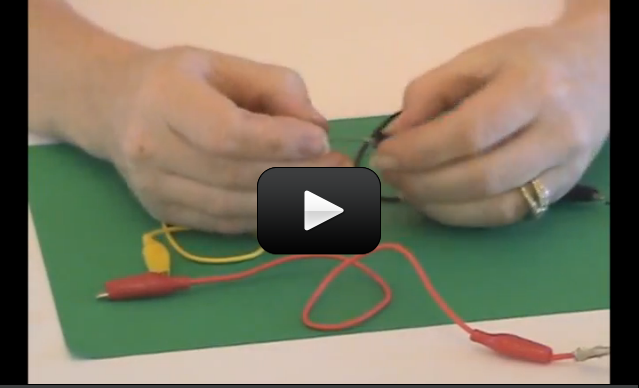
 Make yourself a grab bag of fun things to test: copper pieces (nails or pipe pieces), zinc washers, pipe cleaners, Mylar, aluminum foil, pennies, nickels, keys, film canisters, paper clips, load stones (magnetic rock), other rocks, and just about anything else in the back of your desk drawer.
Make yourself a grab bag of fun things to test: copper pieces (nails or pipe pieces), zinc washers, pipe cleaners, Mylar, aluminum foil, pennies, nickels, keys, film canisters, paper clips, load stones (magnetic rock), other rocks, and just about anything else in the back of your desk drawer.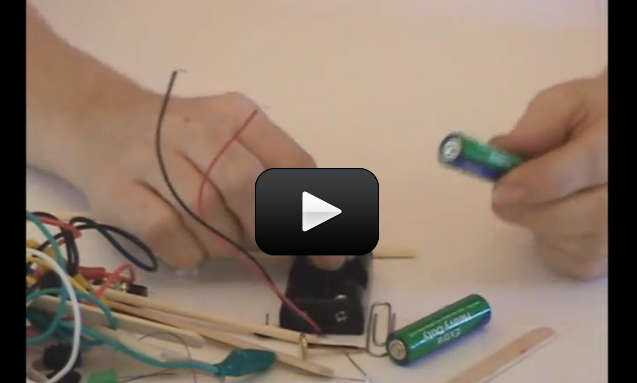
 An electrical circuit is like a NASCAR raceway. The electrons (racecars) zip around the race loop (wire circuit) superfast to make stuff happen. Although you can’t see the electrons zipping around the circuit, you can see the effects: lighting up LEDs, sounding buzzers, clicking relays, etc.
An electrical circuit is like a NASCAR raceway. The electrons (racecars) zip around the race loop (wire circuit) superfast to make stuff happen. Although you can’t see the electrons zipping around the circuit, you can see the effects: lighting up LEDs, sounding buzzers, clicking relays, etc.
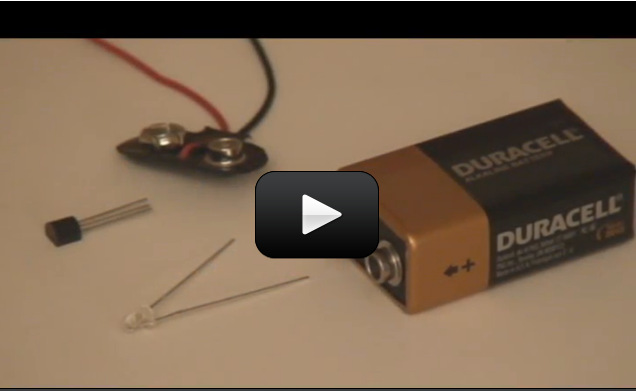
 This simple FET circuit is really an electronic version of the
This simple FET circuit is really an electronic version of the 


 You are actually fairly familiar with electric fields too, but you may not know it. Have you ever rubbed your feet against the floor and then shocked your brother or sister? Have you ever zipped down a plastic slide and noticed that your hair is sticking straight up when you get to the bottom? Both phenomena are caused by electric fields and they are everywhere!
You are actually fairly familiar with electric fields too, but you may not know it. Have you ever rubbed your feet against the floor and then shocked your brother or sister? Have you ever zipped down a plastic slide and noticed that your hair is sticking straight up when you get to the bottom? Both phenomena are caused by electric fields and they are everywhere!