First invented in the 1600s, thermometers measure temperature using a sensor (the bulb tip) and a scale. Temperature is a way of talking about, measuring, and comparing the thermal energy of objects. We use three different kinds of scales to measure temperature. Fahrenheit, Celsius, and Kelvin. (The fourth, Rankine, which is the absolute scale for Fahrenheit, is the one you’ll learn about in college.)
Mr. Fahrenheit, way back when (18th century) created a scale using a mercury thermometer to measure temperature. He marked 0° as the temperature ice melts in a tub of salt. (Ice melts at lower temperatures when it sits in salt. This is why we salt our driveways to get rid of ice). To standardize the higher point of his scale, he used the body temperature of his wife, 96°.
As you can tell, this wasn’t the most precise or useful measuring device. I can just imagine Mr. Fahrenheit, “Hmmm, something cold…something cold. I got it! Ice in salt. Good, okay there’s zero, excellent. Now, for something hot. Ummm, my wife! She always feels warm. Perfect, 96°. ” I hope he never tried to make a thermometer when she had a fever.
Just kidding, I’m sure he was very precise and careful, but it does seem kind of weird. Over time, the scale was made more precise and today body temperature is usually around 98.6°F.
Later, (still 18th century) Mr. Celsius came along and created his scale. He decided that he was going to use water as his standard. He chose the temperature that water freezes at as his 0° mark. He chose the temperature that water boils at as his 100° mark. From there, he put in 100 evenly spaced lines and a thermometer was born.
Last but not least Mr. Kelvin came along and wanted to create another scale. He said, I want my zero to be ZERO! So he chose absolute zero to be the zero on his scale.
Absolute zero is the theoretical temperature where molecules and atoms stop moving. They do not vibrate, jiggle or anything at absolute zero. In Celsius, absolute zero is -273 ° C. In Fahrenheit, absolute zero is -459°F (or 0°R). It doesn’t get colder than that!
As you can see, creating the temperature scales was really rather arbitrary:
“I think 0° is when water freezes with salt.”
“I think it’s just when water freezes.”
“Oh, yea, well I think it’s when atoms stop!”
Many of our measuring systems started rather arbitrarily and then, due to standardization over time, became the systems we use today. So that’s how temperature is measured, but what is temperature measuring?
Temperature is measuring thermal energy which is how fast the molecules in something are vibrating and moving. The higher the temperature something has, the faster the molecules are moving. Water at 34°F has molecules moving much more slowly than water at 150°F. Temperature is really a molecular speedometer.
Let’s make a quick thermometer so you can see how a thermometer actually works:
Please
login or
register to read the rest of this content.






 Flowering plants can be divided into monocotyledons and dicotyledons (monocots and dicots). The name is based on how many leaves sprout from the seed, but there are other ways to tell them apart. For monocots, these will be in multiples of three (wheat is an example of a monocot). If you count the number of petals on the flower, it would have either three, six, nine, or a multiple of three. For dicots, the parts will be in multiples of four or five, so a dicot flower might have four petals, five petals, eight, ten, etc.
Flowering plants can be divided into monocotyledons and dicotyledons (monocots and dicots). The name is based on how many leaves sprout from the seed, but there are other ways to tell them apart. For monocots, these will be in multiples of three (wheat is an example of a monocot). If you count the number of petals on the flower, it would have either three, six, nine, or a multiple of three. For dicots, the parts will be in multiples of four or five, so a dicot flower might have four petals, five petals, eight, ten, etc.



 Broccoli, like all plants, has chlorophyll, making it green. You can really “see” the chlorophyll when you boil broccoli. This is such a simple experiment that you can do this as you prepare dinner tonight with your kids. Make sure you have an extra head of broccoli for this experiment, unless you really like to eat overcooked broccoli.
Broccoli, like all plants, has chlorophyll, making it green. You can really “see” the chlorophyll when you boil broccoli. This is such a simple experiment that you can do this as you prepare dinner tonight with your kids. Make sure you have an extra head of broccoli for this experiment, unless you really like to eat overcooked broccoli.
 If you have a backyard garden, be sure to give it plenty of sunshine, water, and garbage.
If you have a backyard garden, be sure to give it plenty of sunshine, water, and garbage.
 If you’ve ever eaten fruits or vegetables (and let’s hope you have), you have benefited from plants as food. Of course, the plants we eat have been highly modified by growers to produce larger and sweeter fruit, or heartier vegetables.
If you’ve ever eaten fruits or vegetables (and let’s hope you have), you have benefited from plants as food. Of course, the plants we eat have been highly modified by growers to produce larger and sweeter fruit, or heartier vegetables.



















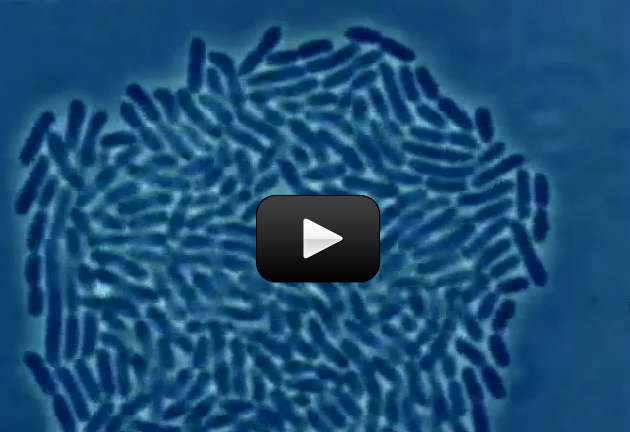
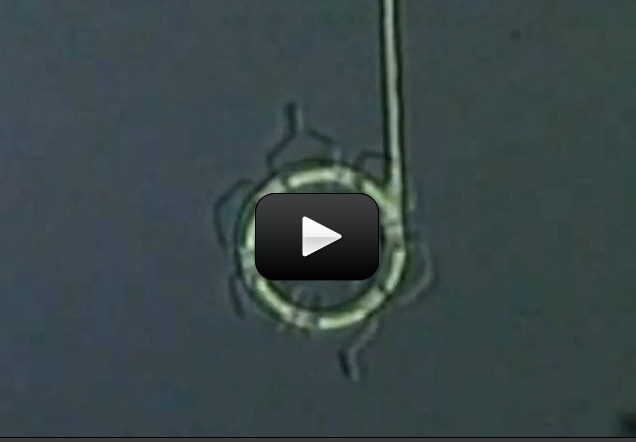
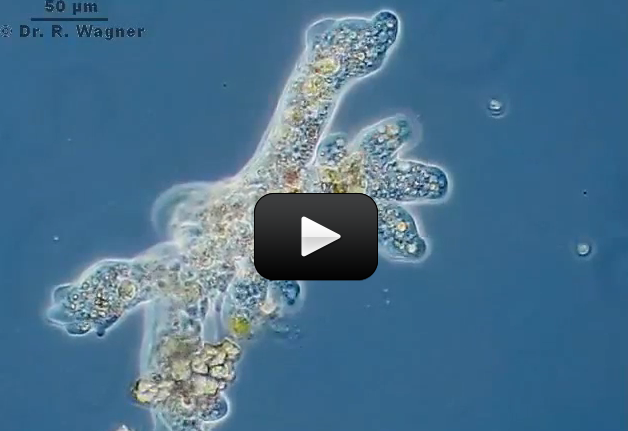
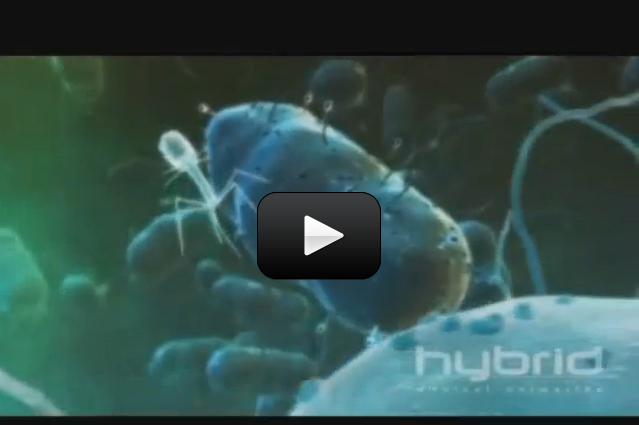
 Some organisms, like bacteria, consist of only one cell. Other organisms, like humans, consist of trillions of specialized cells working together. Even if organisms look very different from each other, if you look close enough you’ll see that their cells have much in common.
Some organisms, like bacteria, consist of only one cell. Other organisms, like humans, consist of trillions of specialized cells working together. Even if organisms look very different from each other, if you look close enough you’ll see that their cells have much in common.

 Photosynthesis is a process where light energy is changed into chemical energy. As we said in the last section, this process happens in the chloroplast of plant cells. Photosynthesis is one of the most important things that happen in cells.
Photosynthesis is a process where light energy is changed into chemical energy. As we said in the last section, this process happens in the chloroplast of plant cells. Photosynthesis is one of the most important things that happen in cells.



 Grab a handful of buttons. Make sure there are all different kinds and colors.
Grab a handful of buttons. Make sure there are all different kinds and colors.