When you hear “roach” you might not immediately think of something that would make a good pet, but not all roaches are like the cockroaches you might have seen in your house!
Species such as the Orange Spotted Roach (Blaptica dubia) make excellent insect pets: they don’t cost much, they have an interesting life cycle and habits, and they do not require much effort to care for. Their average lifespan is about 18 months and you’ll be able to learn more about their fascinating life cycle (from egg to adult) if you allow them to breed!
A pet roach isn’t a pest?
It may seem like all roaches are pests, but of 4,000 species, only 4 or 5 live in homes and are considered pests (such as the American cockroach). Most roaches live in tropical environments far from domesticated areas. They are very different from the kind of household pest you might think of when you hear “roach.”
You might think roaches would make pretty boring pets, but they are surprisingly fast and fun to watch. You can learn a lot about insect anatomy and what makes roaches unique by taking care of them. The species that make good pets do not smell, are not noisy, cannot fly, and generally are very easy to clean up after. They typically are most active at night, because they prefer a dark environment like they have on the floor of the rainforest. They love to hide during the day, but will come out to eat.
Can I touch them? They are meant to be pets, and are perfectly safe to handle. A good environment for roaches is a small aquarium or plastic cage with cardboard egg cartons for them to hang out in. You might try picking up one of the egg cartons where a roach is hiding, then either hold the carton so the roach can crawl around on it or let the roach crawl in to your hands. Hold out your hand, keeping your fingers together and flat. Let the roach crawl on you, then slowly lift out your hand and cup it slightly. Remember to wash your hands afterwards, using warm water and soap. Although these insects don’t cause diseases in humans, they may be carrying harmful bacteria, so it is important to wash your hands so that you don’t get sick.
How long do they live? It varies, but species like Orange Spotted Roaches have a lifespan of 18-24 months. The female gives live birth, usually to 20-30 babies at a time. The babies reach maturity in 3-4 months after they are born. While they are growing into adults, they will molt – shedding their outer hard shell, or exoskeleton, and then growing a bigger one.
Will my roaches breed? If you get one male and one female, there is a good chance that they will breed under the right circumstances. If you do not want baby roaches, keep the temperature of the habitat around 70 degrees, or normal room temperature. Adult Orange Spotted Roaches will be fine at this temperature, but they will not mate because their young need higher temperatures to survive. If you would like to see the complete life cycle, you will need to ensure that their habitat has enough heat and humidity.
Feeding Time: What does a pet roach eat? They are omnivores – they eat plants and meat. So a good basic diet contains protein from plants and animals and fiber from grains. You can buy special roach food for them and then to supplement their diet give them fresh fruits and vegetables once a week. Try putting a slice of apple, banana, orange, carrot, potato, or zucchini, or a few spinach leaves in a shallow plastic dish and put it in their habitat. This will provide vitamins and minerals for your pets. Be sure to take the uneaten produce out of the habitat within 48 hours to prevent mold from growing, or attracting ants or fruit flies. A great roach diet would be dry food every day and a fresh food supplement once a week.
Be sure to keep their water dish full. Roaches can live a long time without food, but usually only survive three days without water. The water dish also helps make their habitat more moist and humid. For easiest care, use water absorbent crystals that hold water. You can keep an airtight container of prepared water crystals in a cool place, and add another crystal to the water dish whenever needed (usually every 2-3 days).
If the habitat is hot and humid, the roaches will be more active, which means they will also eat and drink more.
Cleaning Time: You should periodically clean out your pet roach’s habitat to make sure there is no mold growing. Cleaning out the habitat takes only a few minutes and will prevent any bad odors coming from your insects. When is the right time to clean the habitat? When you see small dark roach droppings starting to collect on the bottom, you should clean the habitat out. Usually about once a month is a good time. The minimum should be once every other month.
To clean out the habitat, first remove the roaches. Place them in a container that has smooth sides to prevent them from climbing out. Pick up the roaches one at a time and transfer them to the carton or other container. If a roach is hiding in an egg carton, carefully lift out the carton, then let the roach crawl off into the container or onto your hand. Wash your hands with soap and warm water after touching the roaches.
Take the food and water dishes out, as well as the egg cartons, and place them on paper towels. Rinse the container out and then wash it with a solution of 10 parts warm water to 1 part bleach. Rinse the container again and dry it thoroughly. Place the food and water dishes back in the container. If the cardboard egg cartons seem clean, put them back into the container. Don’t use foam egg cartons. You can also use cardboard tubes in different sizes (mailing tubes, toilet paper tubes, or wrapping paper tubes cut down to shorter lengths) so the roaches can crawl in them. When you’re finished cleaning, throw the used egg cartons away as well as the paper towels. Transfer your roaches back to their habitat, using a flat hand so they can crawl off.
Building a Roach Ranch: If you decide to get a pet roach, you can create a habitat to be as simple or creative as you like. If you wish to make a more natural-looking habitat for your pet roaches to enjoy, you can buy peat moss or coconut husk mulch from a pet store (in the Reptile section). Put in a layer of moss or mulch (about one inch), then add pieces of bark for the roaches to climb on and hide under. This type of Roach Ranch will be similar to the Orange Spotted Roaches’ natural environment in the rainforests of South America.
You can make a Roach Ranch out of cardboard, which can easily be thrown away when it gets dirty. Make a multi-level mansion for your roaches by cutting 3-4 identical shapes (square, rectangle, L-shape) from cardboard. Put separators in between each level – use stacked cardboard strips that are one inch wide and several inches long. Each level should be separated about ½” or three strips of cardboard stacked together. Use Elmer’s glue to attach the separators and flat levels, and let it dry completely (may take up to 24 hours) before putting it in your roach habitat. Add cardboard tubes or crumpled newspaper to complete your Roach Ranch. Remember that it will be easier to clean if roach droppings can fall freely to the ground. When you clean your habitat, check to see if your Roach Ranch is staying clean. Throw away any parts that have been well-used and add new cardboard material for the roaches to climb.


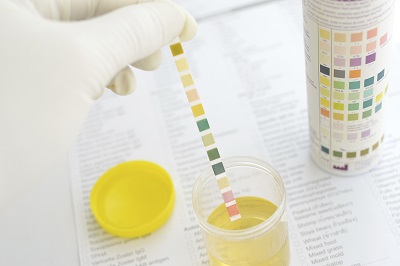
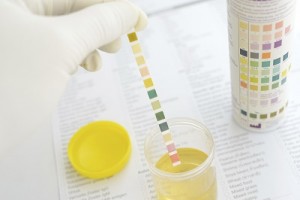 Although urine is sterile, it has hundreds of different kinds of wastes from the body. All sorts of things affect what is in your urine, including last night’s dinner, how much water you drink, what you do for exercise, and how well your kidneys work in the first place. This experiment will show you how the kidneys work to keep your body in top shape.
Although urine is sterile, it has hundreds of different kinds of wastes from the body. All sorts of things affect what is in your urine, including last night’s dinner, how much water you drink, what you do for exercise, and how well your kidneys work in the first place. This experiment will show you how the kidneys work to keep your body in top shape.











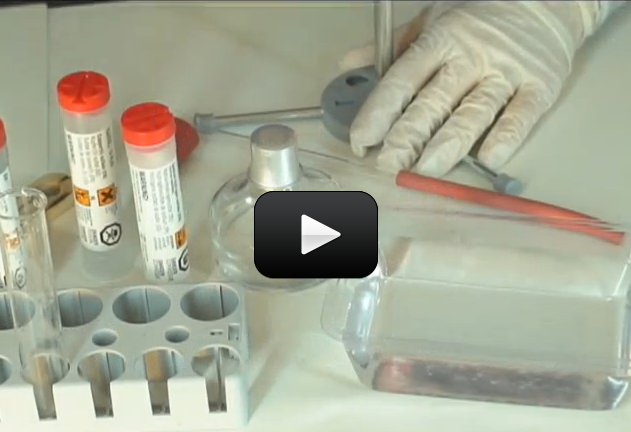



















































 Fossil fuels, which include petroleum, natural gas, and coal, supply nearly 90 percent of the energy needs of the United States and other industrialized nations. Because of their high demand, these nonrenewable energy resources are rapidly being consumed. Coal supplies are expected to last about a thousand years.
Fossil fuels, which include petroleum, natural gas, and coal, supply nearly 90 percent of the energy needs of the United States and other industrialized nations. Because of their high demand, these nonrenewable energy resources are rapidly being consumed. Coal supplies are expected to last about a thousand years.

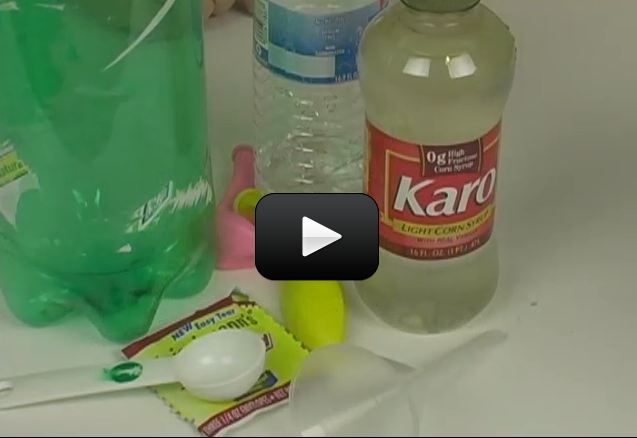

 When birds and animals drink from lakes, rivers, and ponds, how pure it is? Are they really getting the water they need, or are they getting something else with the water?
When birds and animals drink from lakes, rivers, and ponds, how pure it is? Are they really getting the water they need, or are they getting something else with the water?
















