For plants, carbon dioxide enters through the breathing pores on the surface of a plant's leaves (called the stomata), and the water and nutrients enter through the roots. The oxygen gas leaves the leaf through the stomata pores and the sugar (glucose) is distributed to the rest of the plant.
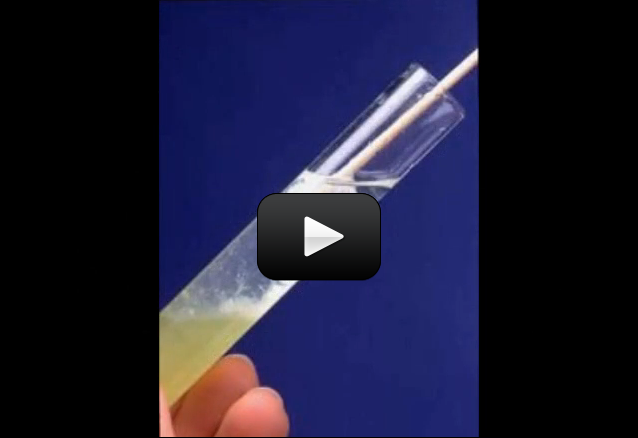
We’re going to measure the rate of photosynthesis of a plant. You basically take small bits of a leaf like spinach, stick it in a cup of water that has extra carbon dioxide in it, and shine a light on it. The plant will take the carbon dioxide from the water and the light from the lamp and make oxygen bubbles that stick to it and lift it to the surface of the water, like a kid holding a bunch of helium balloons. And you time how long this all takes and you have the rate of photosynthesis for your leaf.
Once you’ve got this experiment working, think about other things that might affect the rate of photosynthesis. What about the color of the light (is red better, or yellow, blue, green, UV…?) Does it matter how far the light is from the heat sink? Does the type of leaf matter?
Lab Time:
- Cut out small samples using hole punch
- Place leaves in a cup of water (or use a water-filled syringe)
- Get the leaves to sink by pressing out all air bubbles (plunge syringe several times).
- Add a tsp of a carbon source (like baking soda)
- Place a heat sink on top of the first cup (like another clear cup of water)
- Place in direct sunlight or under a lamp
- Your leave “chads” should rise to the surface if they are generating enough oxygen bubbles!
- Try different leaves, add more/less carbon, change water temp… and have fun!
Additional Notes for the Lab:
Make sure you don’t shine your light directly on the leaf or the glass of water it’s in, or you’ll be adding heat, not just light, to your experiment. Use a clear glass for the main cup of water, and then put a second glass of water on top, and shine your light through the top glass into the lower glass. We don’t want to heat up the water with the leaves because that will change our experiment.
The leaf will absorb energy from the light and convert H2O to make oxygen bubbles. When enough oxygen bubbles are attached to the leaf, the leaf floats to the top and you can time how long it takes that leave to float to the drop from when you first drop it in and switch on the light.
Also, not every kind of leaf is going to work for this experiment. My favorite leaf to use is spinach, but again, go ahead and try different varieties. You know you’ve done it right when the leaves fall to the bottom of the syringe. You can also try pressing the leaf underwater between your thumb and the side of the glass, or leaving it in a dark cupboard overnight to soak in the water. If it still doesn’t sink, discard it and try a different kind of leaf.
Exercises:
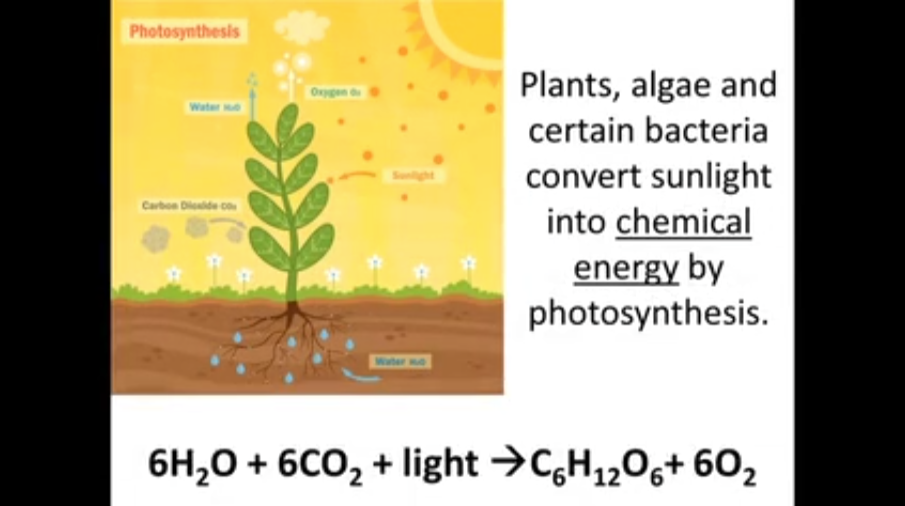
1. Plants, algae and certain bacteria convert sunlight _______________________ into by photosynthesis.
2. Write out what each one of these means in plain everyday words:
6 H2O + 6 CO2 + light --> C6H12O6 + 6 O2








 This experiment is one of my favorites in this acceleration series, because it clearly shows you what acceleration looks like.
This experiment is one of my favorites in this acceleration series, because it clearly shows you what acceleration looks like.























 Gyroscopes defy human intuition, common sense, and even appear to defy gravity. You’ll find them in aircraft navigation instruments, games of Ultimate Frisbee, fast bicycles, street motorcycles, toy yo-yos, and the Hubble Space Telescope. And of course, the toy gyroscope (as shown here). Gyroscopes are used at the university level to demonstrate the principles of angular momentum, which is what we’re going to learn about here.
Gyroscopes defy human intuition, common sense, and even appear to defy gravity. You’ll find them in aircraft navigation instruments, games of Ultimate Frisbee, fast bicycles, street motorcycles, toy yo-yos, and the Hubble Space Telescope. And of course, the toy gyroscope (as shown here). Gyroscopes are used at the university level to demonstrate the principles of angular momentum, which is what we’re going to learn about here.







 Did you know that your cereal may be magnetic? Depending on the brand of cereal you enjoy in the morning, you’ll be able to see the magnetic effects right in your bowl. You don’t have to eat this experiment when you’re done, but you may if you want to (this is one of the ONLY times I’m going to allow you do eat what you experiment with!) For a variation, pull out all the different boxes of cereal in your cupboard and see which has the greatest magnetic attraction.
Did you know that your cereal may be magnetic? Depending on the brand of cereal you enjoy in the morning, you’ll be able to see the magnetic effects right in your bowl. You don’t have to eat this experiment when you’re done, but you may if you want to (this is one of the ONLY times I’m going to allow you do eat what you experiment with!) For a variation, pull out all the different boxes of cereal in your cupboard and see which has the greatest magnetic attraction.

 You are actually fairly familiar with electric fields too, but you may not know it. Have you ever rubbed your feet against the floor and then shocked your brother or sister? Have you ever zipped down a plastic slide and noticed that your hair is sticking straight up when you get to the bottom? Both phenomena are caused by electric fields and they are everywhere!
You are actually fairly familiar with electric fields too, but you may not know it. Have you ever rubbed your feet against the floor and then shocked your brother or sister? Have you ever zipped down a plastic slide and noticed that your hair is sticking straight up when you get to the bottom? Both phenomena are caused by electric fields and they are everywhere!
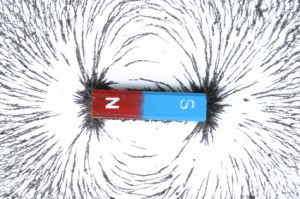 The electromagnetic field is a bit strange. It is caused by either a magnetic field or an electric field moving. If a magnetic field moves, it creates an electric field. If an electric field moves, it creates a magnetic field.
The electromagnetic field is a bit strange. It is caused by either a magnetic field or an electric field moving. If a magnetic field moves, it creates an electric field. If an electric field moves, it creates a magnetic field.



 Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!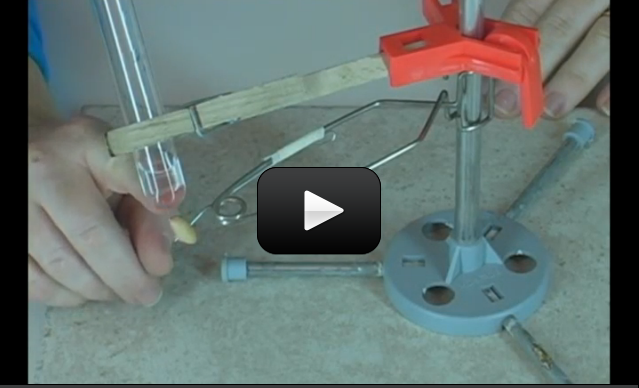


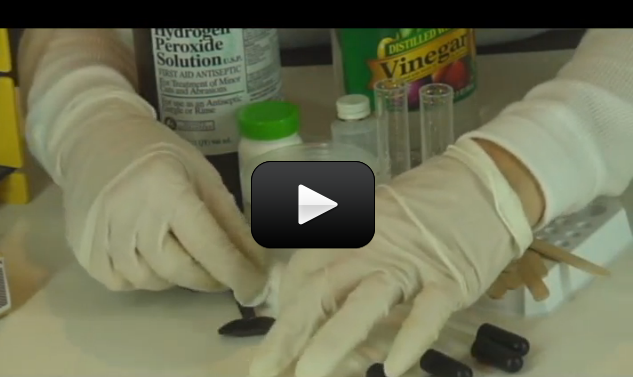










 This experiment below is for advanced students. If you’ve ever wondered why hydrogen peroxide comes in dark bottles, it’s because the liquid reacts with sunlight to decompose from H2O2 (hydrogen peroxide) into H2O (water) and O2 (oxygen). If you uncap the bottle and wait long enough, you’ll eventually get a container of water (although this takes a LOOONG time to get all of the H2O2 transformed.)
This experiment below is for advanced students. If you’ve ever wondered why hydrogen peroxide comes in dark bottles, it’s because the liquid reacts with sunlight to decompose from H2O2 (hydrogen peroxide) into H2O (water) and O2 (oxygen). If you uncap the bottle and wait long enough, you’ll eventually get a container of water (although this takes a LOOONG time to get all of the H2O2 transformed.)
 If you’ve ever owned a fish tank, you know that you need a filter with a pump. Other than cleaning out the fish poop, why else do you need a filter? (Hint: think about a glass of water next to your bed. Does it taste different the next day?)
If you’ve ever owned a fish tank, you know that you need a filter with a pump. Other than cleaning out the fish poop, why else do you need a filter? (Hint: think about a glass of water next to your bed. Does it taste different the next day?)






 So you've played with lenses, mirrors, and built an optical bench. Want to make a real telescope? In this experiment, you'll build a Newtonian and a refractor telescope using your
So you've played with lenses, mirrors, and built an optical bench. Want to make a real telescope? In this experiment, you'll build a Newtonian and a refractor telescope using your 
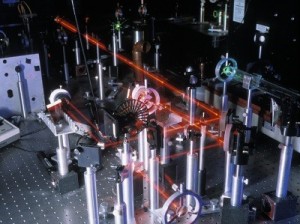 An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).
An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).





 It just so happens that the Sun’s diameter is about 400 times larger than the Moon, but the Moon is 400 times closer than the Sun. This makes the Sun and Moon appear to be about the same size in the sky as viewed from Earth. This is also why the eclipse thing is such a big deal for our planet. You’re about to make your own eclipses as you learn about syzygy.
It just so happens that the Sun’s diameter is about 400 times larger than the Moon, but the Moon is 400 times closer than the Sun. This makes the Sun and Moon appear to be about the same size in the sky as viewed from Earth. This is also why the eclipse thing is such a big deal for our planet. You’re about to make your own eclipses as you learn about syzygy.